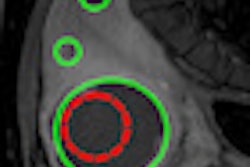
Computer-aided detection (CAD) software developer Medipattern said it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its 3D vascular ultrasound imaging device.
Visualize:Vascular is based on the company's Cadenza algorithm platform. It quantifies image data by segmenting and measuring regions of interest in vessels, helping physicians assess vascular disease using ultrasound imaging readily available in most cardiology, vascular radiology, and vascular surgery practices, Medipattern said.
The device is now available for sale in the U.S., with first shipments anticipated in the second quarter of this calendar year, according to Medipattern.