AuntMinnie.com presents the 11th in a series of columns on the practice of ultrasound from Dr. Jason Birnholz, one of the pioneers of this modality.
Fellow Ultrasounder,
Medicine necessarily involves lifelong learning. We never know enough, yet we have to act, blending our observations and actions with overriding concern for patient well-being. We are bombarded by information, and the barrages intensify with every revision and extension of basic knowledge.
 Dr. Jason Birnholz.
Dr. Jason Birnholz.
I think there is an important role for intuition, which to my way of thinking is a composite of all those things we have seen, heard, or read that linger below the level of immediate conscious recall. Sometimes, these come back when something on the screen triggers a hidden memory.
The summer of 1966 at the end of my junior year in medical school, I was fortunate to do an elective in experimental cardiovascular surgery. One of the projects had to do with boosting cardiac output to overcome congestive failure by the way the left ventricle was paced.
The experimental model of right heart failure involved creating ascites by surgical compression of the thoracic duct in dogs via an upper abdominal incision and placing a ring around the thoracic duct as it passes through the diaphragmatic hiatus just to the right of the aorta. We will come to a case that prompted this recollection after we look into lymph flow dynamics a little more.
The next stop of the memory train is in 1978 in Boston, when I was the radiologist in chief at the Boston Hospital for Women, my most favorite clinical title. I was really lucky to work with Dr. Fred Frigoletto, who is one of the founders of the subspecialty of maternal-fetal medicine, as it is known now, and with Dr. Shirley Driscoll, probably the best known fetal pathologist in the world at that time.
We reported a case of subcutaneous cystic voids in the neck, followed by an amniocentesis diagnosis of monosomy X, i.e., Turner's syndrome, possibly the first example of a specific ultrasound indication of aneuploidy. At the time, we were probably unique in using an electronic sector scanner (phased array) for fetal imaging. As ultrasound imaging quality improved in the early 1980s, there was a flurry of papers on fetal cystic hygromas, which were also thought to indicate Turner's syndrome specifically. All of the imaging of that era was transabdominal.
A landmark publication
By 1985, Dr Beryl Benacerraf, previously my fellow, then my colleague, and subsequently -- and deservedly -- much better known than me, collected and analyzed a combined series of cases with early second-trimester abnormalities of the skin of the neck reported by her, Fred Frigoletto, and Lane Laboda (Beryl's chief technologist). About half of the cases had chromosome problems, of which at least 30% had Down syndrome. This launched all the antenatal ultrasound screening protocols for aneuploidy that we have today. The changes were thought to have something to do with delayed development of lymphatics between 10 and 15 weeks gestational age (GA).
There was considerable controversy about Beryl's findings when they were first reported. Nearly all of the negative comments had to do with poor image quality of the equipment used by her detractors. Of course, when endovaginal scanning became available, there could no longer be any doubt about the generality and value of her work.
NT and screening
Much more recently, these early works were rediscovered in the obstetrics community, mainly using transabdominal imaging, which was pretty much limited to identifying uniform elevation of skin on the back of the neck, i.e., NT or nuchal thickening. A spacing greater than 2 mm or 3 mm is thought to imply about a 50% chance of major aneuploidy.
About 10% of NT cases with normal karyotypes have significant congenital heart disease. This led to the widespread adoption of NT as a screening tool, possibly in conjunction with some maternal serum biomarkers, as a way of triaging elective amniocentesis or chorionic villus sampling (CVS) in addition to the common indication of "age beyond 35 years at delivery" for singletons and 33 years for twins.
When radiology facilities (primarily utilizing endovaginal imaging) were still involved in a big way in this application, there was a distinction on imaging grounds between discrete cystic hygromas and an abnormal NT. Also, many of the cases of either form of abnormality were seen to have small pleural or pericardial effusions or ascites and/or more extensive subcutaneous edema and tended to be classified as "hydrops," which has its own set of causes and pathologic considerations. The peer-reviewed literature has become a confusing jumble of what was really meant by these two different types of neck changes and to what degree either or both of these findings relate to aneuploidy or other genetic conditions.
Case 1: Pregnancy, day 64
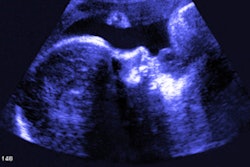
My own private practice has some routine scanning from local physicians and second-opinion scanning from a much wider geographic zone. Most of the "abnormal NT" cases I used to see were just normal embryos resting supine on the amnion. A recent case was referred from an infertility practice: an 11.2-week embryo thought to have an abnormal NT. This embryo, having a higher specific gravity than amniotic fluid, was dependent in the sac, resting on amnion. Here are an endovaginal view of the neck, an energy Doppler view of the heart, and a 3D survey:
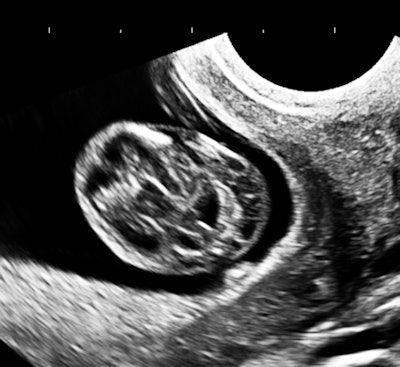 All images courtesy of Dr. Jason Birnholz.
All images courtesy of Dr. Jason Birnholz.
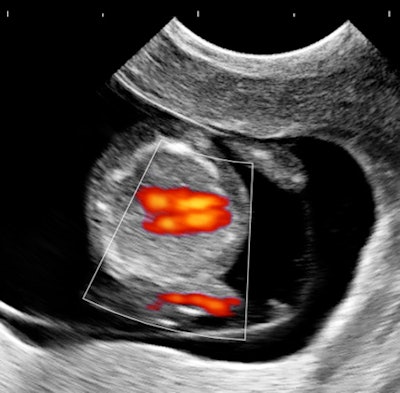
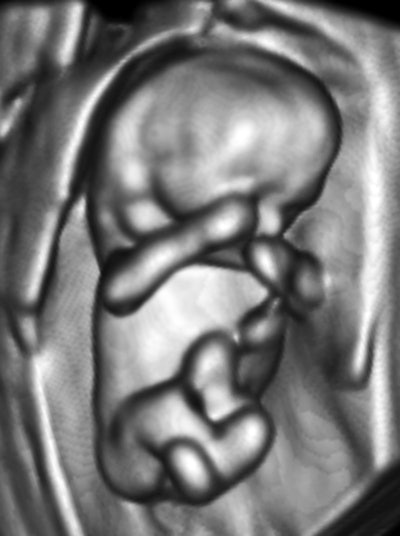
There are multiple cystic hygromas and an apparently normal heart. The 3D really does not look "right" because of a disproportion. No other abnormal findings were evident. We did a cell-free fetal DNA test on maternal blood that established in a few days that this is a case of Trisomy 18.
Recent work has established that when there are neck changes of any type near the start of the second trimester, there is a high likelihood of aneuploidy, but of a wider variety of types than had been thought previously. A cell-free DNA test is a good starting point, but when it is normal, amniocentesis is necessary. When that is also normal, then a microarray (which looks for microduplications and microdeletions) must be done, as this will disclose problems that may have profound developmental deficits, but without other detectable antenatal anatomic findings. A similar protocol should be followed when cardiac defects are found without other findings.
Milky veins
The way to wade through the confusing morass of opinions about NT in lectures and courses is to use the classic and scholarly approach of going to the literature, excluding clinical ultrasound publications. Literature searching and reading is something that anyone can do from any location with Web access. I am going to reference some of the key articles that explain all of our disparate ultrasound observations, and if you want to do some searching yourselves, you might want to use PubMed and try "lymphangiogenesis" or "lymphatic morphogenesis" as key words.
Lymphatics were first described in the 17th century as "milky veins" in the mesentery of a dog after a hearty meal.1 The foundation for understanding NT stems from the landmark work of Florence Sabin more than 110 years ago,2 a long, long time before ultrasound came along. From painstaking ink injection experiments, she observed that endothelial cells bud from cardinal veins to form bilateral jugular sacs from which lymphatics differentiate, including the thoracic duct.
Moving up to modern times, Guillermo Oliver and colleagues3 have shown that lymph sac formation is triggered by the prox1 gene (short for prospero homeobox 1) acting as a transcription factor on endothelial cells from the cardinal veins. This is the linkage of a genetic factor and something that we can observe ultrasonically. Obviously, multiple genes are involved in other steps in lymphatic development. Sac formation starts around six weeks into a pregnancy.
NT is the easiest of the observations to explain. If the thoracic duct and lymphatics form but there is a failure of opening the sacs into the veins (from failed or delayed genetic signaling), there will be diffuse lymphedema in the neck, accompanied by persistence and dilatation of either or both jugular lymphatic sacs. And when you see a real case of NT, you will see the distended sacs as well.
With multiple genes operant in sequence, there is the potential for multiple forms of aneuploidy to present with the same morphologic sign. It is also pretty common to find persistent jugular lymphatic sacs without any nuchal skin edema. This seems to be a single gene defect; it is not associated with aneuploidy, but it is still important and should prompt a microarray before deciding that it is an innocuous finding.
The fetal circulation is a low pressure one, i.e., high heart rate and small stroke volume. Heart failure in embryos and fetuses is the diastolic type. High pressure in the jugular veins, preventing lymph drainage, will also prompt NT in the first and early second trimesters, which is why there is such a high incidence of congenital heart problems identified in cases with NT and euploidy.
What about the timing of nuchal signs?
There can be transient, minimal nuchal skin elevation seen at 10 to 12 weeks GA, typically not after 16 weeks and not particularly associated with any problems. True pathologic nuchal findings have their genesis early, but they persist and get worse instead of going away. Nevertheless, there is a popular notion that "screening" should be done around 12 weeks and presumably expands upon the concept of Professor John Dobbing4 that each organ system has a time window of extreme vulnerability to injury.
The potentially transient nature of nuchal skin changes early in pregnancy is explained by the elegant micrographic studies of the early human fetus by Professor V.M. Petrenko and her colleagues.5 These show the development of smooth muscle and valves in lymphatics beginning in the second trimester with progressive functional maturation subsequently. The physiology of lymphatic development is actually pretty complex and very interesting. Recently, there have been models of "lymphangions" as functional units for achieving one-way propulsion of tissue fluid into the thoracic duct.6
The real spectrum of NT
Simple "screening" has tended to focus on low-resolution imaging and just "NT." When imaging is performed routinely with high-resolution (low-noise) endovaginal imaging, there is a more frequent finding of multiple discrete subcutaneous cystic abnormalities in the neck than NT. These can be found over a wide gestational age range, and they have a higher association with significant genetic abnormalities.
Simple NT involves the thoracic duct either being absent or present but not communicating with the venous system. With multiple cystic hygromas, it is the smaller lymphatics that are malformed, independent of the thoracic duct. About 30 separate genes have already been identified that are involved in lymphangiogenesis and in the morphogenesis, remodeling, and maturation of lymphatics,6 so it is really no wonder at the range of genetic problems that may be associated with this one family of appearances in antenatal ultrasound exams.
Newton's aphorism that "nature abhors a vacuum" is fundamental in physics and chemistry. For the life sciences, we need that remember that "nature loves redundancy." Our task as diagnostic imagers is to stop looking for separate magic signs of whatever, and, instead, try to understand the multiplicity of mechanisms that converge into what we see. And, of course, for NT screening, viewing should always be done from the portal that provides the best quality images, which is almost always an endovaginal one.
A great case
About the same time that we saw the first case, an adult male patient was referred for a vague reason that seemed to have something to do with morbid obesity and failure to lose weight, except through the use of diuretics. He had been hospitalized a few months earlier with pneumonia. The patient was very pleasant but quite enormous, and I really worried that I would not be able to see much with my equipment.
However, as soon as the probe touched the right upper quadrant (I tend to start everything with the liver), it was obvious that there was massive ascites with no increase in abdominal wall or other fat. The fluid was echo-free, and there were no peritoneal studs. The liver and kidneys appeared normal, hepatic veins were patent and normal in caliber, and a brief cardiac survey was normal as well. In a few minutes of scanning, there was a major finding that was a complete surprise and most of its most serious causes were eliminated from consideration. I just love the diagnostic power of ultrasound, especially in a completely exploratory role.
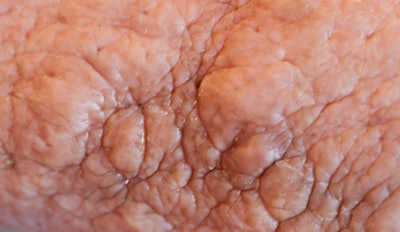
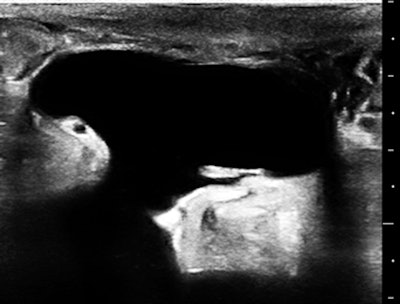
The skin of the lower abdomen is bubbly from lymphedema, and there was a large subcutaneous, paraumbilical sac that opened into the peritoneal cavity.
Remember the lab dogs with surgical ascites? If you had been diligent in your reading about lymphatics, you would have come across the fact that the most common cause of ascites globally is filariasis, afflicting more than 100 million people where mosquitoes are infested with Wuchereria bancrofti and Brugia malayi. The parasites gain entry into superficial lymphatics and propagate centrally, leaving occluded channels in their migratory wake.
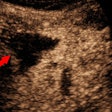
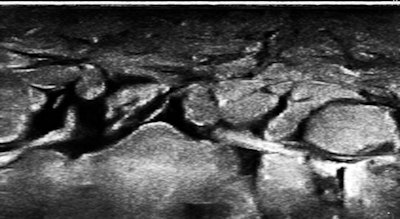
So, of course, we had a look at the thoracic duct, which had an abnormal appearance (image below). The technique of visualizing the terminal portion of the thoracic duct, adjacent to the left jugular vein, was published by Seeger et al (Radiology, September 2009, Vol. 252:3, pp. 897-904).
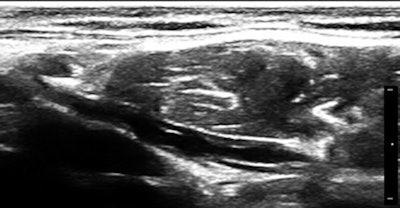
Filariasis usually afflicts legs or genitals, ascending from infections acquired from walking barefoot through an infested area. Our patient happened to be an armorer and professional rifleman who had used a prone firing position when instructing in the tropics, hence the anterior lower abdominal entry of the parasites.
A final word
When we start any imaging education for ultrasound, x-rays, and radionuclides, we try to cope with the complexity of what we do by looking for specific signs or the magical pathognomonic finding. However, bodies and disease just do not work that way. As you gain understanding and acquire mastery, you begin to see the commonalities and interrelated nature of all we encounter, not the differences. It is tapping that reserve of intuition that enables you to cope with the apparent diversity of what you see every day, like these two cases that seem so entirely different, but which dangle from the same branchlet of the pathophysiologic tree.
References
- Asellius G. De lactibus sive lacteis venis. Milan: J.B. Bidellium; 1627.
- Sabin FR. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am J Anat. 1902;1(3):367-389.
- Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, et al. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120(11):2340-2348.
- Smart JL, Dobbing J, Adlard BPF, Lynch A, Sands J. Vulnerability of developing brain: relative effects of growth restriction during the fetal and suckling periods on behavior and brain composition of adult rats. J Nutr. 1973;103:1327-1338.
- Petrenko VM, Gashev AA. Observations on the prenatal development of human lymphatic vessels with focus on basic structural elements of lymph flow. Lymphat Res Biol. 2008;6(2):89-95.
- Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011;193(4):607-618.
Dr. Jason Birnholz is a graduate of Johns Hopkins School of Medicine and did his diagnostic radiology residency at Massachusetts General Hospital. He was awarded an advanced academic fellowship from the James Picker Foundation and has been a professor of radiology and obstetrics. He is a fellow of the American College of Radiology and the Royal College of Radiology.
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnie.com, nor should they be construed as an endorsement or admonishment of any particular vendor, analyst, industry consultant, or consulting group.