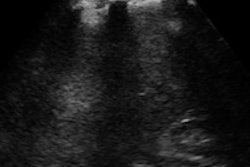
Ultrasound technology developer SuperSonic Imagine announced that its Aixplorer MultiWave ultrasound system has received U.S. Food and Drug Administration (FDA) clearance for the quantification capabilities of its ShearWave elastography (SWE) technology.
Aixplorer's UltraFast imaging platform images up to 200 times faster than conventional ultrasound, simultaneously imaging and computing the velocity of the generated waves, the firm said. In addition, its electronic palpation displays real-time quantitative (kPa) tissue elasticity on a color-coded map.
ShearWave elastography also provides evaluation of multifocal stiff tissue, dynamic analysis of elasticity changes, and longitudinal follow-up of tissue abnormalities and treatment, SuperSonic Imagine said.
The quantitative ShearWave elastography protocol will be exhibited at the Society of Radiologists in Ultrasound (SRU) meeting in October. In November, it will be exhibited at the American Association for the Study of Liver Diseases (AASLD) meeting and at RSNA 2013.