Screening for hepatocellular carcinoma (HCC) in patients with chronic liver disease can detect early-stage cancer, but it's unclear whether systematic screening would yield a survival advantage over clinical diagnosis, according to an analysis published on Tuesday in the Annals of Internal Medicine.
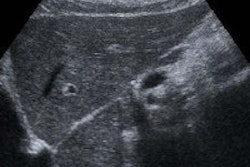
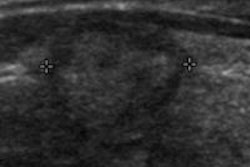
The review included 22 studies in the literature and assessed the utility of methods such as ultrasound and serial alpha-fetoprotein screening. The team of researchers, led by Dr. Devan Kansagara of Portland Veterans Affairs Medical Center, found that "the body of evidence on which current recommendations for screening are based has substantial shortcomings."
The research project was commissioned by the U.S. Veterans Health Administration.
Controversial recommendations
A number of professional societies recommend using imaging methods and tumor markers for HCC screening, primarily in patients at higher risk due to chronic hepatitis B or cirrhosis; however, these recommendations remain controversial due to concerns over the quality and paucity of existing evidence to support screening, and the potential of overdiagnosis and patient harms, according to the researchers.
To better understand the incremental benefits and harms of routine HCC screening compared with clinical diagnosis, the study team conducted a systematic review of the literature. They searched Medline, PsycInfo, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and ClinicalTrials.gov from the inception of the databases to June 2013. They later updated the Medline, PsycInfo, and ClinicalTrials.gov searches in April 2014 (Ann Intern Med, June 17, 2014).
Kansagara and colleagues included all English-language controlled clinical trials and observational studies that assessed the effects of screening on HCC-specific and all-cause mortality in adults. Studies also had to include a comparison group of patients who did not have routine screening. Observational studies that did not consider important confounding factors were excluded. Using a tool developed by the Cochrane Collaboration, two reviewers independently assessed each included trial's quality, and disagreements were resolved through discussion.
Two reviewers also graded the strength of evidence for outcomes by using published criteria that considered a body of evidence as well as the internal validity of individual studies, according to the researchers. In addition, existing tools were adapted to evaluate the quality of observational studies.
Low-strength evidence
Of the 286 potentially relevant studies gathered from the literature search, 22 studies met the group's inclusion criteria. Of these 22, two trials and 18 observational studies provided only very-low-strength evidence regarding the effect of HCC screening on mortality.
The researchers concluded that the two trials -- both conducted in China -- had substantial methodological flaws that threatened their internal validity, and their findings have limited applicability beyond the hepatitis B patient population. They also noted, though, that the observational studies, most of which included patients with cirrhosis and hepatitis B, hepatitis C, or alcoholic liver disease, showed that screening detects patients with earlier-stage disease and who more frequently receive curative therapy.
"However, it is impossible to say whether the longer survival in patients with screen-detected disease was a true effect of screening or reflects lead- and length-time biases inherent to all observational studies, as well as selection biases that were common in many of the studies," they wrote.
Two additional trials compared ultrasound screening intervals: One was a Taiwanese study that compared four-month and 12-month screening intervals in patients with serologic evidence of hepatitis B or C. Despite more patients in the four-month screening interval group having early-stage disease, survival rates were similar among both screening intervals. Furthermore, the "study had an unclear risk of bias because of poor reporting of outcome assessment and statistical analyses," they wrote.
The other trial, which had a low risk of bias, compared three-month and six-month ultrasound screening intervals, and found similar all-cause mortality rates among both groups.
Observational studies
The 18 observational studies compared survival in patients with screening-detected HCC and those diagnosed with HCC incidentally as part of another workup or because of symptoms. The researchers noted that patients in these studies who had undergone screening had earlier-stage HCC than those who hadn't received screening. They also determined that more screened patients received potentially curative treatment, and survival was generally higher among screened patients.
However, several methodological considerations temper confidence for drawing conclusions from these studies, according to the researchers.
"Most of the studies were single-center retrospective cohort studies in which all patients with diagnosed HCC were first identified and screening status was subsequently determined," they wrote. "Few studies reported data on loss to follow-up, and many did not report using a comprehensive method to assess mortality outcomes equally in screening and nonscreening groups."
In addition, selection bias was a concern for 15 of the studies, and none of the studies reported any direct harms of screening or examined the psychological effects of screening, the authors noted.
Kansagara and colleagues concluded that there is very-low-strength evidence from which to draw conclusions about the effects of HCC screening on mortality in high-risk patients with chronic liver disease.
"Screening can identify more patients with earlier-stage disease who are candidates for potentially curative treatments, but there is limited evidence from which to draw firm conclusions about the balance of health outcome benefits and harms of using routine screening to identify HCC," the researchers wrote.
They noted, though, that their findings neither support nor refute current policy recommendations for HCC screening.