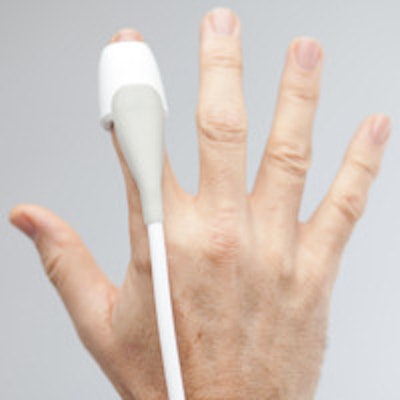
Ultrasound needs a little more of a human touch. That's the concept behind SonicEye, a fingertip-worn ultrasound probe developed by Oregon-based start-up Sonivate Medical. The company is moving SonicEye to market with significant backing from the U.S. Department of Defense.
Created by miniaturizing the components of standard ultrasound transducer technology, SonicEye is placed on a clinician's fingertip, enabling ultrasound to be more intuitive and easier to perform than when conducted with traditional transducers held in a pinch-grip, according to President and CEO David Starr.
"If you put an ultrasound probe on a fingertip, you really leverage your natural brain-hand-finger connection," Starr said.
Sonivate's initial SonicEye model features a 7.5-MHz linear-array probe and has received U.S. Food and Drug Administration (FDA) clearance for use with vascular access and needle-guidance procedures. Other versions are also being developed for emergency medicine and ob/gyn applications.
Technology at your fingertips
Sonivate was founded in 2001 by Dr. Ronald Schutz, a cardiologist who took a strong interest in ultrasound as part of his clinical practice.
"He noticed when he was supporting the cardiac surgeons through transesophageal echocardiography that they were facile with their fingers, and decided he wouldn't have to be called into the operating room to do the echo for them if he was able to put a probe on their fingertip," Starr told AuntMinnie.com. "That was the inspiration for the technology."
The company really began to gain momentum about four years ago, and it has been buoyed by more than $5 million in grant funding from the U.S. Department of Defense, according to Starr. The firm will also soon close on a $1.6 million financing round from angel investors, GeekWire.com reported.
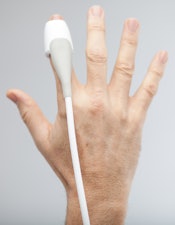 The SonicEye fingertip ultrasound probe. Image courtesy of Sonivate.
The SonicEye fingertip ultrasound probe. Image courtesy of Sonivate.Anesthesiologists and radiologists who perform needle-guided procedures appreciate SonicEye because they are able to wear the finger probe on their nondominant finger, Starr said. This allows them to "image and palpate and perform the procedure with their own two hands with greater precision, stability, and control than traditional ultrasound." With traditional methods, another person may need to hold the probe, or the physician may be forced to awkwardly hold the probe while doing the procedure, he added.
There has been a strong preference so far for the finger-worn probe over traditional ultrasound in procedures such as nerve blocks, vascular access, biopsies, and joint injections, Starr said.
Overall, SonicEye probes function the same as existing ultrasound probes and can work with existing ultrasound scanners, provided that Sonivate's cables are compatible with their systems.
"[The ultrasound vendor] might need to adjust software to recognize our probe, but for the most part it's just like adding a third-party probe to an ultrasound system," Starr said.
SonicEye is also being used to train medical students at several universities, including Oregon Health & Science University in Portland and Western University of Health Sciences in Pomona, CA.
After seeing interest in its finger-probe technology from emergency medicine physicians, Sonivate also developed a SonicEye prototype that utilizes a 3-MHz biplane phased-array probe suitable for performing general imaging and the focused assessment with sonography for trauma (FAST) exam, Starr said. Sonivate is seeking FDA clearance for this model.
The company is also working to modify SonicEye for use in ob/gyn applications, allowing imaging and physical exams to be performed simultaneously, Starr said. An intraoperative probe may also be developed in the future.
Military use
As a longtime supporter of Sonivate, the U.S. Department of Defense believes in the benefits of fingertip-worn ultrasound probes, not just for combat casualty care, but also for its traditional healthcare activities, according to Starr.
"They saw the clear advantage of our technology in enabling more point-of-care ultrasound use, and for making ultrasound easier to use so it can be used by more and more clinicians, shortening the learning curve for ultrasound and enabling traditional or nonusers of ultrasound today to be using ultrasound in the future," he said.
The high-frequency, linear-array probe for needle-guided procedures was funded by the military primarily for hospital applications, such as procedures performed by trauma surgeons, anesthesiologists, and emergency physicians, among others.
The version of SonicEye with the biplane phased-array transducer was funded by the military for use in combat casualty care, in particular for Special Forces combat medics to carry with them for use at the point of injury.
"This offers several advantages," Starr said. "It's very easy and intuitive to use, and you could slip it under body armor or a shock blanket due to its low form factor. It also really lends itself toward telemedicine use; even if the clinician can't interpret the image, they can easily acquire the image and send it to an interpreting physician telemedically."
In November 2013, Sonivate inked a master Cooperative Research and Development Agreement (CRADA) with the U.S. Army Medical Research and Materiel Command. Madigan Army Medical Center in Tacoma, WA, and Brooke Army Medical Center in San Antonio entered into multiyear CRADAs with Sonivate and agreed to provide collaborative research focusing on comparative assessment of linear-array ultrasound probe technology for vascular access and breast biopsies, respectively.
Google Glass
The military is also interested in the possibility of pairing the fingertip-probe technology with heads-up displays like Google Glass. Six years ago, the U.S. military provided Sonivate with a couple of prototypes of heads-up displays that could be plugged into the video port of the ultrasound system.
"These heads-up displays allow the clinician -- without moving their head -- to see the ultrasound image in one eye and see what's going on with the patient and what they're doing with the other eye," he said. "And then they become fused in a very exquisite way, so that in this augmented reality they are able to see and do without moving their head. [This] is important because when you are doing a procedure that requires precision, when you move your head [to look at the monitor] you also move your hands."
Today, the commercially available Google Glass offers an additional advantage of having an outward-facing camera that lends itself well to telemedicine applications, he said.
"A clinician in some remote location can see the patient and also see the image while the clinician onsite can see the image while they're caring for the patient," he said.
Sonivate demonstrated the pairing of SonicEye with Google Glass at the recent American Society of Echocardiography (ASE) annual meeting.
There are some challenges, however, to implementing Google Glass with SonicEye, according to Starr.
"It's not optimally suited for 'fusing' the image in the eye while the clinician is looking at the patient, due to the way the projection in the glasses fits on the eye and sits on the nose," he said. "Secondly, everything is transmitted via the cloud, which creates some latency that is not acceptable when you're doing a procedure on your own."
But all of these challenges could easily be remedied by Google in the future, he noted.
"These medical applications could be a huge opportunity for Google, in particular with these needle-guided procedures in which the ideal would be to replace the display on the ultrasound system with something lightweight and easy like Google Glass," he said. "It could potentially reduce cost and also improve performance of the overall procedure because it enables the clinician not to have to move their head."
Commercial activities
Sonivate has a distribution agreement in place with Fukuda Denshi, which is currently selling SonicEye in the U.S. in a number of markets, primarily to physicians who perform a lot of needle-guided procedures with small-parts imaging, Starr said.
SonicEye is also drawing clinical interest for neonatal and pediatric use, Starr said.
"The clinician can actually hold and calm the baby while imaging at the same time," he said. "That's very comforting for the baby as well as the parent."
Sonivate is also in discussions with several other ultrasound companies that want to make their systems compatible with the probe and distribute it to their customers. The company is primarily looking for OEM channels, but it may also sign on distributors later this year.
Though it's currently focusing on the U.S. market, Sonivate is also developing plans for other parts of the world, Starr said.
In addition to developing a family of clinical finger ultrasound probes to address a variety of clinical applications, Sonivate plans to form a number of OEM partnerships with ultrasound companies.
"Where we're really going in our product direction -- and the end user is demanding it -- is to go to a wearable, wireless ultrasound system," he said. "Ultimately, you can imagine a finger probe and it's connected by a short wire or cable to a wearable ultrasound system that could be the size of a smartphone. That could then transmit to a heads-up display, and that is the ultimate embodiment of the technology, because it enables clinicians to be untethered from the system and cable-free."




















