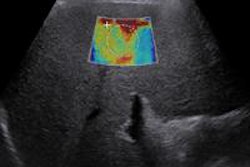
Ultrasound contrast developer Acusphere has voluntarily withdrawn a regulatory application in Europe for its Imagify ultrasound contrast agent for the detection of coronary artery disease.
On August 28, Acusphere announced that it had pulled Imagify's Marketing Authorization Application (MAA) submitted to the European Medicines Agency's Committee for Medicinal Products for Human Use.
The withdrawal of the MAA in Europe does not affect the status of Imagify's special protocol assessment with the U.S. Food and Drug Administration (FDA), the company said.