Yielding comparable tumor measurements and accuracy, contrast-enhanced ultrasound (CEUS) can be a viable alternative to contrast-enhanced MRI for assessing breast cancer response to neoadjuvant chemotherapy, according to research published in the May issue of the Journal of Ultrasound in Medicine.
In a pilot study, a research team led by Dr. Sandy Lee of the University of Southern California's Keck School of Medicine found that CEUS measurements of tumor size correlated well with MRI, and both modalities produced the same accuracy for predicting how the tumors were responding to therapy. What's more, CEUS tumor measurements correlated better with pathologic tumor size than did MRI tumor measurements.
"CEUS may be a good alternative exam when MRI cannot be performed in the neoadjuvant chemotherapy setting," Lee told AuntMinnie.com.
An alternative to MRI?
MRI is commonly used to evaluate treatment response in breast cancer patients undergoing neoadjuvant chemotherapy. Although MRI is a great tool for this job, the modality can't be used in certain patients, such as those with renal disease, a pacemaker, or an allergy to gadolinium contrast agents, according to Lee.
 Dr. Sandy Lee from the University of Southern California.
Dr. Sandy Lee from the University of Southern California.The researchers sought to investigate the use of contrast ultrasound because it's easy to perform, has no radiation, and can be used in patients with contraindications for MRI. In addition, microbubble contrast agents have a relatively safe profile, Lee said.
In the prospective pilot study, CEUS and contrast-enhanced MRI were performed on 30 patients with invasive breast cancer lesions larger than 2 cm. Of the 30 patients, 29 had invasive ductal carcinoma and one had metaplastic carcinoma. Both imaging studies were performed before neoadjuvant chemotherapy and afterward prior to surgery (J Ultrasound Med, May 2017, Vol. 36:5, pp. 901-911).
All contrast ultrasound exams were performed using the Definity ultrasound contrast agent (Lantheus Medical Imaging) on an Epiq ultrasound system (Philips Healthcare) with a 12-MHz linear transducer or a 9-MHz curvilinear transducer, depending on the tumor size and depth. Postprocessing analysis of the CEUS data was performed at a later time using Qlab software (Philips). Blinded to the MRI results and surgical data, a dedicated breast imager with more than 15 years of experience compiled the tumor measurements.
When possible, the MRI and CEUS studies were performed on the same day. However, sometimes the exams were performed on different days due to patient scheduling limitations, according to the researchers.
Patients received dynamic contrast-enhanced MRI using a gadolinium-based contrast agent and either an Excite HD 1.5-tesla scanner (GE Healthcare) or a Symphony 1.5-tesla system (Siemens Healthineers) with a dedicated breast coil. The MRI protocol included axial and coronal short-tau inversion recovery images, a precontrast T1-weighted acquisition, and additional postcontrast T1-weighted acquisitions obtained five to seven minutes after contrast administration.
Postprocessing was performed with CADstream software (Merge Healthcare) to generate subtractions and dynamic time/signal intensity curves. Two dedicated breast imagers -- blinded to the CEUS and surgical data -- then calculated tumor measurements.
The researchers compared the CEUS and MRI results with the pathologic tissue specimens that were obtained from definitive surgical treatment, which is usually performed at their institution four to six weeks after the completion of neoadjuvant chemotherapy. Spearman coefficients (r values) were calculated to quantify the correlation between imaging and pathologic findings. The higher the r value, the higher the level of agreement.
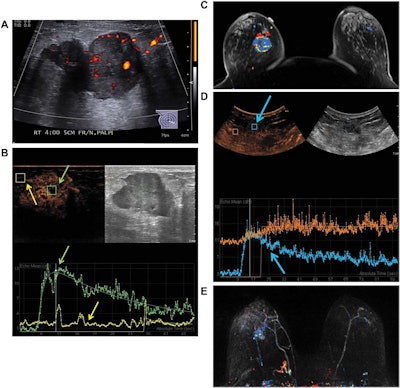
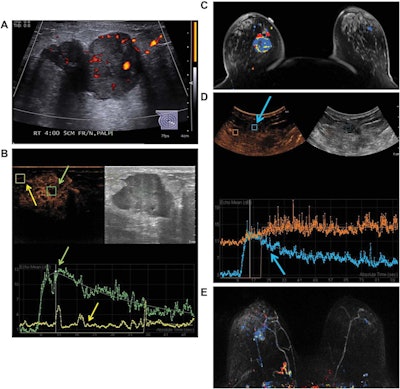
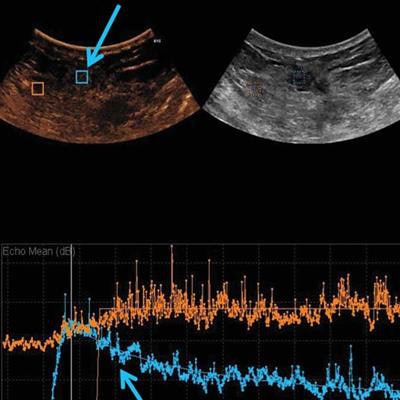
Images are from a 50-year-old woman presenting with a right breast lump that has biopsy-proven infiltrating ductal carcinoma. A: Targeted right-breast ultrasound imaging showed an oval hypoechoic mass with indistinct margins measuring up to 3.7 cm. B: At baseline before starting neoadjuvant chemotherapy, CEUS showed an avidly enhancing tumor (green arrows) with tumor peak intensity on the time-intensity curve generated by the Qlab software. The region of interest was placed on adjacent normal breast tissue (yellow arrows), which had a flat line on the time-intensity curve. The y-axis of the time-intensity graph corresponds to the intensity or mean echo (decibels), whereas the x-axis corresponds to the absolute time (seconds). C: Axial postcontrast fat-saturated MRI at baseline showed an enhancing tumor with mixed kinetics in the right breast. After neoadjuvant chemotherapy was completed but before surgery, contrast-enhanced and MRI scans were again performed. D: CEUS showed a 2-cm residual enhancing tumor with a time-intensity curve (blue arrows) that had rapid enhancement and washout. E: Maximum-intensity projection contrast-enhanced MRI performed after neoadjuvant chemotherapy was completed showed a decrease in the size of tumor to 2 cm with predominantly progressive kinetics. At the time of right lumpectomy, this patient had 1.5 cm of invasive tumor in the right breast, consistent with a noncomplete pathologic response. Both the contrast-enhanced ultrasound and MRI studies predicted that there would be noncomplete pathologic response at the time of surgery. Images courtesy of the Journal of Ultrasound in Medicine.
Strong agreement
At baseline prior to treatment, contrast ultrasound and MRI both yielded a 3.1-cm median tumor size; the modalities had strong correlation (r = 0.88, p < 0.001) in tumor size measurements. The researchers noted that one patient had a deep tumor that did not enhance on baseline CEUS, but it was visible on conventional ultrasound and displayed subthreshold enhancement on MRI.
"The deep location and low vascularity of the tumor likely contributed to its lack of enhancement on the contrast-enhanced US scan," the authors wrote.
After neoadjuvant chemotherapy, agreement on tumor size dropped (r = 0.66, p = 0.004) but was still comparable. The researchers observed, however, that in a subset of 15 patients who had both CEUS and MRI studies that could be compared with tumor size at surgery, CEUS (r = 0.75, p < 0.001) correlated better with tumor size at surgery than MRI did (r = 0.42, p = 0.095).
"Contrast-enhanced US findings correlate well with MRI findings and may be even more accurate in predicting residual tumor size after [neoadjuvant chemotherapy]," the authors wrote.
In addition, contrast ultrasound and MRI were equally effective in predicting pathologic response to treatment. The modalities both accurately predicted three (75%) of the four patients who had a complete pathologic response, and eight (72.7%) of the 11 patients with a noncomplete pathological response.
While CEUS performed well in the study, the researchers noted that MRI has its own advantages.
"It can evaluate the remainder of the same breast and the contralateral breast at the same time, while CEUS is more limited to evaluating the known tumor," Lee said.
CEUS may also not be a good exam for evaluating small or deep tumors in the breast, she said.
Future work
The researchers acknowledged that their study was limited by the small number of patients. Studies with a larger patient cohort are now needed to further investigate the use of CEUS in the neoadjuvant chemotherapy setting, according to Lee.
The group is also exploring the possibility of using CEUS to determine when ultrasound-guided breast biopsies may be necessary, she said.