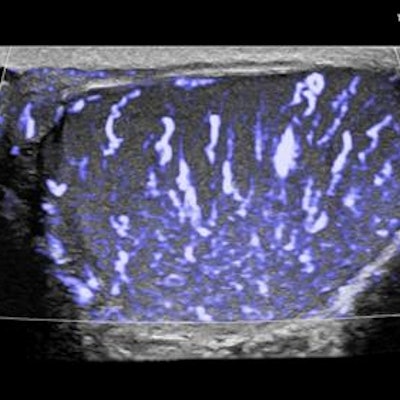
The U.S. Food and Drug Administration (FDA) has given 510(k) marketing clearance to the eL18-4 transducer for small-parts imaging from Royal Philips, the parent company of Philips Healthcare.
The eL18-4 transducer is part of Philips' Ultimate Small Parts Solution package for small-parts imaging. The package consists of four devices that together are capable of detecting musculoskeletal injuries and irregularities in small organs such as the breasts, testicles, and thyroid. Each component offers a distinct feature:
- The eL18-4 PureWave linear-array transducer provides detailed resolution through fine-elevation focusing.
- MicroFlow Imaging enables assessment of blood flow in small vessels.
- Elastography quantifies tissue stiffness.
- Precision Biopsy enhances needle visualization during biopsy.
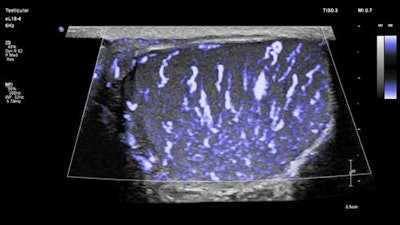 The eL18-4 ultrasound transducer is designed for small-parts imaging. Image courtesy of Philips.
The eL18-4 ultrasound transducer is designed for small-parts imaging. Image courtesy of Philips.The new package bolsters clinicians' capacity to evaluate and treat small parts through an easy-to-perform exam, according to Vitor Rocha, business leader of the ultrasound group at Philips.
Philips plans to introduce the complete package, currently available on its Epiq 7 and 5 and Affiniti 70 ultrasound scanners, at the upcoming World Federation for Ultrasound in Medicine and Biology (WFUMB) congress in Taipei, Taiwan.

















