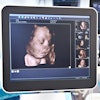
Artificial intelligence (AI) software that combines deep-learning and machine-learning techniques can measure carotid intima-media thickness (CIMT) more accurately than sonographers can, according to research in the July 1 issue of Computers in Biology and Medicine.
In testing, a multi-institutional and multinational team led by Mainak Biswas of the National Institute of Technology Goa in India found that its deep learning-based model outperformed previous automated methods. The model was also up to 20% more accurate than sonographers in measuring CIMT, an important biomarker for cardiovascular disease and stroke monitoring.
"The results showed that the performance of the [deep learning]-based approach was superior to the nonintelligence-based conventional methods that use spatial intensities alone," the authors wrote. "The [deep-learning] system can be used for stroke risk assessment during routine or clinical trial modes."
An important biomarker
An increase in CIMT -- the mean perpendicular distance between the lumen-intima (LI) and the media-adventitia (MA) interfaces -- has been associated with an increased risk of cardiovascular events and stroke. However, the current process of measuring CIMT suffers from accuracy and reproducibility issues due to factors such as variability in patient nationality, ethnicity, disease, and age group. Technical challenges also play a role; traditional manual segmentation of these regions is slow, error-prone, and subject to intraobserver and interobserver variability, according to the researchers (Comput Biol Med, July 1, 2018, Vol. 98, pp. 100-117).
As a result, a number of automated techniques have been developed for predicting CIMT, using various spatial features such as grayscale median, pixel classification, gradient edges, space-scale, or a combination of these features, the researchers explained.
"Despite their strong contributions, these external factors make the spatial-based methods prone to variability and a lack of robustness when it comes to completely automated designs," they wrote.
Biswas and colleagues hypothesized that a deep-learning system would be more reliable and accurate than previous methods, thanks to its inherent ability to provide better regional segmentation output. To train and test a deep-learning model, the researchers used 396 high-resolution B-mode ultrasound images of the right and left common carotid artery from 203 patients at Toho University in Japan. The ultrasound scans were obtained on one of three ultrasound systems (Aplio XV, Aplio XG, and Xario) from Canon Medical Systems. Of the 396 images, 90% were used for training the deep-learning model and 10% were set aside for testing.
Manual tracing of the lumen and adventitia borders was performed using ImgTracer software (AtheroPoint). Dr. Jasjit Suri, PhD, from AtheroPoint served as senior author on the study.
A 2-stage system
The researchers developed a two-stage system that made use of both deep and machine learning. In the first stage, a convolution layer-based encoder was used to extract image features, while a decoder based on a fully convolutional neural network (CNN) performed image segmentation. The raw inner lumen borders and raw outer interadventitial borders generated during this process were then smoothed with a machine learning-based method. The model utilized these final borders to calculate CIMT values from the LI and MA far walls using the standardized polyline distance metric method.
As two different sets of gold standards -- lumen regional information and interadventitial regional information -- were used during the design of the deep-learning model, the researchers trained and evaluated two different algorithms. Compared with the gold standard, the two deep-learning algorithms yielded CIMT error rates of 0.126 ± 0.134 mm and 0.124 ± 1.0 mm. They also significantly outperformed previously developed systems for measuring CIMT, according to the researchers.
Biswas and colleagues also compared the performance of the deep-learning algorithms with mean far-wall CIMT measurements calculated by sonographers in 346 images. Both models correlated better with the ground truth than the sonographer measurements, which had been performed in real-time in the institution's vascular ultrasound laboratory.
| Coefficient of correlation with ground truth | |||
| Manual sonographer measurement of CIMT | Deep-learning method | Improvement of deep-learning method over sonographer measurement | |
| Ground truth 1 | 0.80 | Model 1: 0.96 | 20% |
| Ground truth 2 | 0.83 | Model 2: 0.95 | 14.5% |
The deep-learning method takes only a few milliseconds to perform, according to the researchers. They acknowledged, though, that the system relied on a dataset limited to a Japanese diabetic cohort, and it has not been tested on a wide variety of datasets.
As a result, it needs to be evaluated further in a multiethnic patient population with subclinical atherosclerosis and low-, moderate-, and high-risk scenarios, they wrote. The approach must also be evaluated on ultrasound images from different equipment vendors, and it should also be extended from a desktop PC-based application to a web-based version, according to the group.