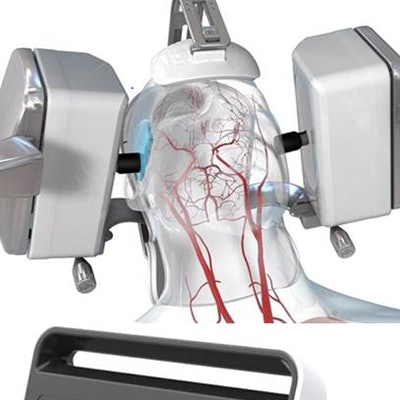
Medical robotics company Neural Analytics has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its NeuralBot robotic assistance technology.
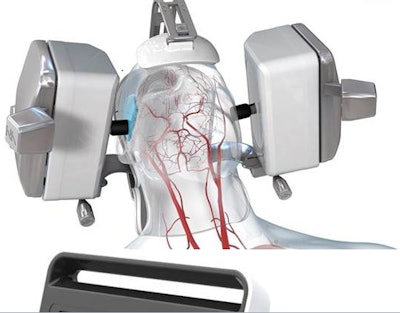 Lucid robotic ultrasound machine. Image courtesy of Neural Analytics.
Lucid robotic ultrasound machine. Image courtesy of Neural Analytics.NeuralBot is an accessory designed to automatically adjust the orientation of Neural Analytics' Lucid M1 transcranial Doppler (TCD) ultrasound unit, which can noninvasively monitor cerebral blood-flow velocities within the head and neck. Working in tandem with Lucid M1, NeuralBot can help clinicians characterize blood flow in a patient's brain as well as diagnose various neurological disorders.
The company will immediately offer NeuralBot together with its Lucid M1 TCD device as a single unit, called the Lucid Robotic System.



















