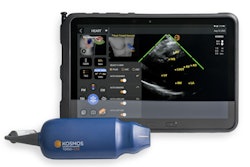
Ultrasound artificial intelligence (AI) technology developer EchoNous has added continuous-wave and pulsed-wave Doppler capabilities to its Kosmos handheld ultrasound platform.
Kosmos was cleared by the U.S. Food and Drug Administration in March 2020. The new capabilities add to the scanner's high performance at a lower price and with high mobility for use by cardiologists and other clinician users, according to the company.
Kosmos may be easily upgraded for the continuous and pulsed-wave Doppler capabilities, EchoNous said.