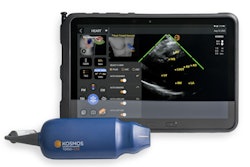
EchoNous has received clearance from the U.S. Food and Drug Administration (FDA) for its Lexsa linear probe for the company's Kosmos portable ultrasound scanner.
 The Lexsa 128-channel linear ultrasound probe. Image courtesy of EchoNous.
The Lexsa 128-channel linear ultrasound probe. Image courtesy of EchoNous.Lexsa is 128-channel linear probe and enables point-of-care full-body imaging on Kosmos. Due to its capabilities for extremity imaging, Lexsa is expected to become a critical ultrasound tool for central line placement, nerve blocks, and guiding injections into joints, according to EchoNous.