Ultrasound ablation potentially improves motor function and reduces dyskinesia in patients with Parkinson's disease, according to research published February 23 in the New England Journal of Medicine.
A team led by Dr. Vibhor Krishna from the University of North Carolina found that compared with a sham treatment, unilateral pallidal ultrasound ablation yielded a higher percentage of patients with such improvements over a three-month period, though it was also linked to adverse events.
Previous open-label studies have shown that unilateral ultrasound ablation can result in reduced motor symptoms in patients with Parkinson's disease. These were performed on the globus pallidus, a subcortical structure of the brain.
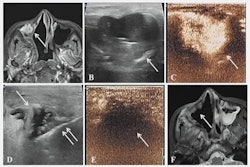
This method does not require an incision and involves real-time guidance with MRI to monitor tissue temperature while ultrasound beams are delivered to gradually heat target areas.
Krishna and colleagues wanted to test the safety and efficacy of focused ultrasound ablation in their study. They randomly assigned patients with Parkinson's disease in an off-medication state into a 3:1 ratio to either undergo either focused ultrasound ablation or a sham procedure. Ablation was performed opposite of the most symptomatic side of the body.
The team included 94 patients in their study, 69 of whom underwent ultrasound ablation, and 25 who underwent the sham procedure. However, 65 patients who underwent ablation and 22 patients who underwent the sham procedure completed the primary-outcome assessment.
The researchers found that 45 patients (69%) in the ablation group had a response compared to seven (32%) in the sham group (p = 0.003).
They also looked at how patients fit into two sets of criteria. These included the Movement Disorders Society-Unified Parkinson's Disease Rating Scale, part III (MDS-UPDRS III) for the treated side in the off-medication state, and the Unified Dyskinesia Rating Scale (UDysRS) in the on-medication state. Of the patients in the active-treatment group who had a response, 19 met the MDS-UPDRS III criterion only, eight met the UDysRS criterion only, and 18 met both criteria.
The team also reported that results for secondary outcomes were "generally" in the same direction as those of the primary outcome. For example, 30 out of 39 patients in the ablation group who had a response at three months continued to have a response at 12 months. However, 11 pallidotomy-related adverse events rated as severe or life-threatening in this group were recorded, compared with one in the control group. Such events included dysarthria, gait disturbance, loss of taste, visual disturbance, and facial weakness.
The study authors called for longer and larger trials to find out the effect and safety of ultrasound ablation in patients with Parkinson's disease.