Clarius Mobile Health has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new Clarius Bladder AI, a noninvasive tool that automatically measures bladder volume.
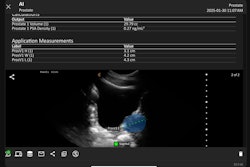
 Clarius Bladder AI measures bladder volume and provides real-time feedback to clinicians. It recently received 510(k) clearance from the FDA. Image courtesy of PR Newswire.
Clarius Bladder AI measures bladder volume and provides real-time feedback to clinicians. It recently received 510(k) clearance from the FDA. Image courtesy of PR Newswire.
The tool is now available in the U.S. with the Clarius PAL HD3, Clarius PA HD3, and the Clarius C3 HD3 wireless handheld ultrasound scanners. It provides real-time feedback to clinicians, with the company highlighting its uses in monitoring urinary retention and assessing bladder emptying in patients with neurogenic bladder or urinary tract obstruction.
Current Clarius members with eligible scanners will be able to use Clarius Bladder AI on their Clarius scanners today, the company said.