Some nonmass breast lesion characteristics on ultrasound exams can be considered suspicious for malignancy at screening ultrasound, according to research published November 5 in Radiology.
A team led by Su Min Ha, MD, PhD, from Seoul National University Hospital in South Korea found that the following nonmass lesion features on ultrasound can be considered suspicious: calcifications, posterior shadowing, segmental distribution, and mixed echogenicity. It also found that having a negative mammogram was associated with a lower malignancy rate and lower positive predictive values for sonographic features compared with having an abnormal mammogram.
“These sonographic features can help detect clinically occult malignant nonmass lesions at screening ultrasound with high interreader agreement,” the Ha team wrote.
While breast nonmass lesions are typically seen at screening and diagnostic ultrasound, there is limited knowledge about imaging features of these lesions. Ha and colleagues sought to identify these features and determine which ones are suspicious for malignancy based on mammographic findings.
The retrospective, multicenter study included data collected from 2012 to 2019 from 993 women with an average age of 50 years. Of the 993 nonmass lesions found in these women, 914 were benign and 79 were malignant.
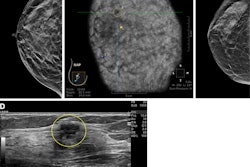
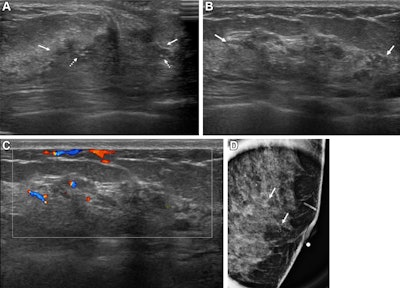 Images depict a 43-year-old woman with a malignant nonmass lesion that was diagnosed as microinvasive ductal carcinoma. (A) Transverse and (B) longitudinal ultrasound images show a hypoechoic nonmass lesion (solid arrows) with segmental distribution in the left breast. The lesion has associated calcifications (dashed arrows in A). (C) Longitudinal color Doppler ultrasound image shows high vascularity. (D) Magnified image in the mediolateral oblique view from screening mammography shows grouped calcifications (arrows), which are considered a mammographic correlate of the ultrasound-detected nonmass lesion (A radiopaque marker attached to the skin is visible in the image because the mammographic exam was performed after screening breast ultrasound). Ultrasound-guided core needle biopsy and pathologic examination revealed ductal carcinoma in situ, but the lesion was upgraded to microinvasive ductal carcinoma at subsequent surgery (2.5-cm ductal carcinoma in situ, histologic grade II, estrogen receptor-positive, progesterone receptor-positive, human epidermal growth factor receptor type 2-negative).RSNA
Images depict a 43-year-old woman with a malignant nonmass lesion that was diagnosed as microinvasive ductal carcinoma. (A) Transverse and (B) longitudinal ultrasound images show a hypoechoic nonmass lesion (solid arrows) with segmental distribution in the left breast. The lesion has associated calcifications (dashed arrows in A). (C) Longitudinal color Doppler ultrasound image shows high vascularity. (D) Magnified image in the mediolateral oblique view from screening mammography shows grouped calcifications (arrows), which are considered a mammographic correlate of the ultrasound-detected nonmass lesion (A radiopaque marker attached to the skin is visible in the image because the mammographic exam was performed after screening breast ultrasound). Ultrasound-guided core needle biopsy and pathologic examination revealed ductal carcinoma in situ, but the lesion was upgraded to microinvasive ductal carcinoma at subsequent surgery (2.5-cm ductal carcinoma in situ, histologic grade II, estrogen receptor-positive, progesterone receptor-positive, human epidermal growth factor receptor type 2-negative).RSNA
The team observed that malignant nonmass lesions have larger average sizes than benign lesions (2.6 cm vs. 1.9 cm, p < 0.001).
On multivariable analysis, the following sonographic features as measured by odds ratios (ORs) were tied to malignancy: calcifications (OR, 21.6, with 1 as reference; p < 0.001), posterior shadowing (OR, 6.9; p < 0.001), segmental distribution (OR, 6.2; p < 0.001), mixed echogenicity (OR, 5.0; p < 0.001), and size (OR, 1.5; p = 0.01).
The researchers also found the following:
- Calcifications, posterior shadowing, segmental distribution, and mixed echogenicity showed positive predictive values (PPVs) of 44%, 22%, 22.9%, and 16.6%, respectively.
- Having a negative mammogram was associated with a lower malignancy rate (2.8% vs. 28.8%) and lower PPVs for sonographic features (0.7% to 10.4% vs. 24% to 55%) than having a positive mammogram.
- Sonographic features achieved good to excellent interreader agreement (Fleiss κ 95% CI lower bound range, 0.63 to 0.81).
The study authors noted that since malignancy rates and predictive values of sonographic features differ according to whether women have a positive or negative mammogram, a combined assessment is needed to determine a threshold for biopsy.
In an accompanying editorial, Lars Grimm, MD, from Duke University in Durham, NC, wrote that the study is an important step in establishing the term "nonlesion mass" in clinical practice. However, he cautioned that this success will “depend heavily” on the next edition of the BI-RADS atlas.
“There is clear interest from the radiology community, and all signs point to inclusion in some form,” Grimm wrote. “Once the official green light has been given, further work is needed to test these descriptors in more diverse settings, especially in other countries with different population demographics and ultrasound usage patterns.”
Grimm also called for prospective studies to determine clinical management strategies for different nonmass lesion descriptors, especially in combination with multimodality imaging.
The full study can be found here.