Dear Women's Imaging Insider,
In May 2006, the U.S. Food and Drug Administration proposed to make it easier to get full-field digital mammography systems to market by loosening its requirements for product regulatory submissions. Three years later, the mammography industry is still waiting for the agency to follow through.
Why hasn't the FDA finalized its own recommendation? That's the question we ask in this month's Women's Imaging Insider. We interviewed industry experts to investigate the issue and received a range of interesting responses. Click here to read the experts' hypotheses. As an Insider subscriber, you have access to the article well before the rest of our members.
As for other news in your Women's Imaging Digital Community, read what Massachusetts researchers are saying about how routine mammograms prevent deaths from breast cancer. The study findings were presented Tuesday at a press briefing for the 2009 Breast Cancer Symposium, which is being held this week in San Francisco -- check back for our coverage from the conference.
Also be sure to check out the following stories:
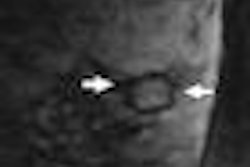
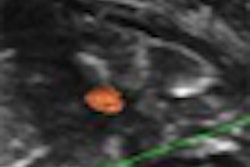
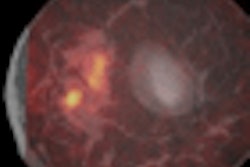
- Learn what New York University researchers are saying about whether breast MRI can help with equivocal mammographic findings.
- Read international editor Eric Barnes' report from the American College of Radiology Imaging Network (ACRIN) fall meeting on mammography versus MRI for presurgical planning.
- Get the nitty-gritty on how hackers have compromised the personal data of women involved in a mammography research study at the University of North Carolina at Chapel Hill.
As always, if you have a comment, report, or article idea to share about any aspect of women's imaging, please feel free to contact me at [email protected].