AI could help reduce interval breast cancer rates through screening digital breast tomosynthesis (DBT) exams, according to findings published July 29 in Radiology.
A team led by Manisha Bahl, MD, from Massachusetts General Hospital in Boston, found that an AI model correctly localized about one-third of interval cancers on retrospective evaluation of screening DBT exams.
“Our study adds to the growing body of literature about the potential impact of AI on the optimization of screening mammography outcomes,” Bahl and colleagues wrote.
While using DBT in clinical practice may boost screening performance, the researchers noted a lack of long-term outcome data for the modality. Previous studies suggest that combining DBT with 2D digital mammography does not significantly reduce false-negative rates compared to mammography alone.
And while AI continues to be explored for detecting false-negative cancers, the team noted a lack of data focusing on AI’s potential in finding false-negative cancers at screening DBT.
Bahl and colleagues studied the performance of an AI model (Insight DBT, Lunit) for finding and localizing interval cancers at screening DBT. They also validated the diagnostic threshold of the model by analyzing its performance across the following DBT exams: interval, true positive, true negative, and false positive.
The team had the algorithm retrospectively analyze screening DBT exams collected between 2011 and 2023. It marked lesions on DBT sections by assigning a score between 0 and 100, with scores of 10 or higher considered positive. Two breast radiologists independently reviewed the AI-positive exams.
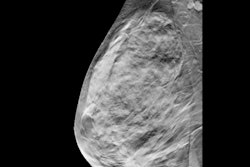
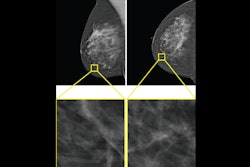
 Example of an interval cancer retrospectively detected by an AI algorithm. A 41-year-old woman presented for a screening digital breast tomosynthesis (DBT) examination; its findings were interpreted as negative. Ten months later, the patient presented with a lump in the left breast and was subsequently diagnosed with grade 3 invasive ductal carcinoma. At retrospective evaluation of the initial screening
Example of an interval cancer retrospectively detected by an AI algorithm. A 41-year-old woman presented for a screening digital breast tomosynthesis (DBT) examination; its findings were interpreted as negative. Ten months later, the patient presented with a lump in the left breast and was subsequently diagnosed with grade 3 invasive ductal carcinoma. At retrospective evaluation of the initial screening
mammogram, the AI algorithm marked a suspicious lesion (white outline) in the left breast, with high scores of 81 on the craniocaudal view and 75 on the mediolateral oblique view. This area of architectural distortion corresponds to the cancer that was subsequently diagnosed.RSNA
The final analysis included 224 interval cancers found in 224 women with an average age of 61 years. The algorithm correctly localized 73 interval cancers (32.6%) upon retrospective evaluation of screening DBT exams.
The investigators found that larger size at surgical pathology (37 mm) and axillary lymph node positivity (41.3%) were significantly associated with interval cancers detected by AI. For undetected cancers, the team reported that lesion size was 22 mm while axillary lymph node positivity was 22.8%.
Using the same threshold, AI correctly localized 84.4% of true-positive cancers and correctly categorized 85.9% and 73.3% of true-negative and false-positive cases as negative, respectively.
The study authors highlighted their results toward improving outcomes from screening DBT. They also called for future studies to examine AI’s impact on false-negative rates and other performance metrics, such as false-positive rates.
“Furthermore, to realize the reduction in interval cancer rates shown in this study, the radiologist must review and agree with the AI output to recall the patient for further imaging,” they added.
The results provide compelling evidence of AI’s potential to improve breast cancer screening, according to an editorial written by Christoph Lee, MD, from the University of Wisconsin-Madison, and Hannah Milch, MD, from the University of California, Los Angeles.
They added that realizing AI’s promise in the clinic requires prospective trials, continued algorithm refinement, and focused training for radiologists who use AI for image interpretation.
“Only through these deliberate and collaborative efforts can we ensure AI integration genuinely improves patient outcomes while minimizing unnecessary harms,” Lee and Milch wrote.
The full study can be found here.