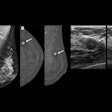
A deep-learning (DL) model can improve the detection of breast arterial calcifications (BACs), according to research published June 20 in Academic Radiology.
A team led by Sabee Molloi PhD, from the University of California, Irvine, highlighted the performance of its DL model, which successfully classified, segmented, and quantified BACs found on mammograms. It highlighted that these findings may advance cardiovascular risk screening.
“This study supports the potential for integrated dual-purpose screening in women’s healthcare,” the Molloi team wrote.
BACs in recent years have gained recognition as an indicator of cardiovascular disease risk. This makes improving detection and quantification methods important, with mammography at the fore.
However, current analysis methods for BACs are limited. Manual approaches can be time-consuming and observer-dependent, and current automation methods are inconsistent.
“Perhaps most critically for quantification, achieving precise segmentation of BAC boundaries has remained a persistent challenge, hindering accurate measurement,” the researchers wrote.
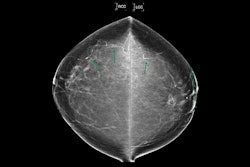
Molloi and co-authors developed their DL model with the goal of detecting, segmenting, and quantifying BAC in mammograms. Their goal included improving mammographic screening for cardiovascular risk assessment. The model employed a modified U-Net architecture that incorporates Hausdorff loss (for better finding BAC boundaries), Dice loss, and binary cross-entropy loss for segmentation and later quantification.

Seven readers manually annotated mammograms used for the model’s development and from there, placed women into six BAC mass categories for the validation set: 0 mg (no detectable BAC), 0 to 20 mg, 20 to 40 mg, 40 to 60 mg, 60 to 80 mg, and over 80 mg. These categories reflect a clinically relevant range of BAC severity, the team wrote.
The team tested the model’s performance on a retrospective dataset using mammograms from 369 women. The distribution of patients by BAC level, however, included 160 women in the training set and 40 women in the validation set.
The team reported the following findings:
The model achieved high segmentation accuracy with Dice scores of 0.90 for the training set and 0.89 for the validation set.
Bland-Altman analysis (which measures agreement between measurements) validated the model’s quantification reliability, showing a mean difference of −0.98 mg of calcium in the training set.
The model achieved high classification accuracy with F1 scores of 0.97 and 0.93 for validation and training sets, respectively, in finding BACs.
The study authors highlighted the model’s potential in streamlining radiology workflows, reducing human error, and being used in future studies that integrate BAC data with clinical risk profiles.
“This can potentially lead to earlier and more accurate identification of cardiovascular risk factors in women undergoing routine mammographic screening,” they wrote.
The team further highlighted that the model “holds the potential to transform mammographic screening into a dual-purpose tool for both breast cancer and cardiovascular risk assessment.”
The full study can be read here.