Contrast-enhanced mammography (CEM) could be considered for screening women when added to screening digital breast tomosynthesis (DBT) exams, suggest findings published June 17 in Radiology.
Researchers led by Wendie Berg, MD, PhD, from the University of Pittsburgh School of Medicine in Pennsylvania found that CEM significantly improved breast cancer detection after screening DBT. It improved overall performance despite also increasing false-positive rates.
“CEM seems very promising as an alternative to MRI and is better tolerated and easier to implement, though it is not yet FDA approved for screening,” Berg told AuntMinnie.com.
Many state laws require insurance coverage for supplemental screening MRI in women at elevated risk for breast cancer, such as women with dense breasts. However, MRI’s capacity is limited by higher costs and other issues tied to patient access. This has led to many women instead receiving a supplemental ultrasound.
CEM in previous studies has shown its potential to be on par with breast MRI in this setting. The Berg team studied the impact of CEM when added to DBT in women eligible for screening MRI.
The prospective study, which took place in 2021 and 2022, included 601 women who completed their CEM exams. Two radiologists interpreted each imaging study, where reader one recorded DBT findings first and reader two recorded CEM findings first.
Of the total women, 12 were diagnosed with 16 malignant lesions. Reader one detected cancers in five of these women, while reader two detected one lesion at DBT. Both observers also found these lesions via CEM.
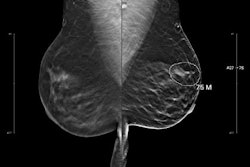
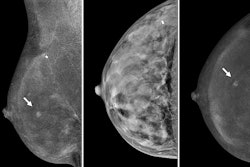
 Images depict a 61-year-old woman with heterogeneously dense breasts and a family history of breast cancer in her mother, who was diagnosed at age 65. The participant’s estimated lifetime risk of breast cancer was 15% according to Tyrer-Cuzick, version 8.0. (A) Craniocaudal (left) and mediolateral oblique (right) mammograms show heterogeneously dense breast tissue, which may obscure detection of small masses; these appear normal even in retrospect. (B) Craniocaudal (left) and mediolateral oblique (right) recombined images from contrast-enhanced mammography (CEM) obtained following intravenous injection of 100 mL of iopamidol 370 show a high-conspicuity enhancing round mass in the upper outer left breast (arrows), assessed as BIRADS 4B by both readers. (C) Targeted transverse (left) and sagittal (right) ultrasound images show an indistinctly marginated, round, hypoechoic, 7-mm mass in the 1 o’clock position of the left breast, 3 cm from the nipple (arrows) that appeared to correspond to the CEM finding. Ultrasound-guided core biopsy and excision revealed a 1.1-cm, grade 1 invasive ductal carcinoma with ductal carcinoma in situ that was estrogen receptor positive and progesterone receptor and human epidermal growth factor 2 negative, with a Ki-67 proliferation index of 15%. Two sentinel nodes were negative.RSNA
Images depict a 61-year-old woman with heterogeneously dense breasts and a family history of breast cancer in her mother, who was diagnosed at age 65. The participant’s estimated lifetime risk of breast cancer was 15% according to Tyrer-Cuzick, version 8.0. (A) Craniocaudal (left) and mediolateral oblique (right) mammograms show heterogeneously dense breast tissue, which may obscure detection of small masses; these appear normal even in retrospect. (B) Craniocaudal (left) and mediolateral oblique (right) recombined images from contrast-enhanced mammography (CEM) obtained following intravenous injection of 100 mL of iopamidol 370 show a high-conspicuity enhancing round mass in the upper outer left breast (arrows), assessed as BIRADS 4B by both readers. (C) Targeted transverse (left) and sagittal (right) ultrasound images show an indistinctly marginated, round, hypoechoic, 7-mm mass in the 1 o’clock position of the left breast, 3 cm from the nipple (arrows) that appeared to correspond to the CEM finding. Ultrasound-guided core biopsy and excision revealed a 1.1-cm, grade 1 invasive ductal carcinoma with ductal carcinoma in situ that was estrogen receptor positive and progesterone receptor and human epidermal growth factor 2 negative, with a Ki-67 proliferation index of 15%. Two sentinel nodes were negative.RSNA
The radiologists found cancers in the other six women through CEM only, equating to an incremental cancer detection rate of 10 per 1,000 women. Five of these women had invasive disease, with three cases being lobular. The researchers also highlighted that the false-positive rate of combined DBT plus CEM was 21.6% for reader one, an increase of 13.4% over DBT alone (8.1%).
But even with the increased false-positive rate, the CEM approach improved the overall area under the curve for reader one from 0.73 (DBT alone) to 0.92 (p = 0.016).
Of the entire study population, reader one recommended biopsy for 50 women (8.3%). Six of these women had a resulting cancer diagnosis. At the lesion level, the team reported a positive predictive value of biopsies performed (PPV3) of 11% for biopsies brought on with CEM only by reader one. Finally, the team reported no interval cancers at one-year follow-up.
Berg said the results add to growing evidence of CEM’s utility, such as that of the United Kingdom-based Breast screening Risk Adapted Imaging for Density (BRAID) study.
“Unlike the BRAID trial, however, we were comparing with tomosynthesis and in women undergoing annual screening,” she said. “The ongoing CMIST [Contrast-enhanced Mammography Imaging Screening Trial] trial has a similar design to ours.”
The team is next studying whether annual DBT and screening MRI or CEM would be enough for screening in women with a personal history of breast cancer, citing the small size of found cancers (median, 0.7 cm) and the lack of interval cancers in the current study. Berg said the next study is being supported by the Breast Cancer Research Foundation.
“We need more studies like that in broader populations, as patient access and tolerance of MRI are quite limiting to widespread use in screening and CEM holds great promise,” she told AuntMinnie.
The full study can be accessed here.
Disclosure: Berg also serves as chief scientific advisor to DenseBreast-info.org. The study received financial support from the Pennsylvania Breast Cancer Coalition.