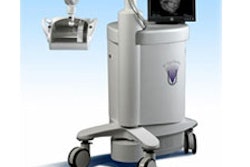
Direct Medical Systems (DMS) of San Ramon, CA, has submitted an application to the FDA for clearance to market its M3000 mammography system.
DMS imports medical imaging equipment through a joint venture between a manufacturing company in China and the People's Republic of China.
Upon FDA clearance, DMS plans to market the system domestically through a U.S. distributor and worldwide through a network of distributors.
By AuntMinnie.com staff writers
November 29, 2005
Related Reading
DMS nets FDA nod, April 15, 2005
Copyright © 2005 AuntMinnie.com