In the 2000 epic film "Gladiator," an enslaved military general named Maximus led a motley band of fighters-for-hire. In their first Coliseum battle, Maximus and his men formed a tight huddle while their "enemies" circled around them. By banding together, the gladiators had the proverbial strength in numbers and prevented their opponents from picking them off, one by one.
In a way, the mammography community is in the same position as Maximus and crew. But instead of being hunted by an Amazon warrior driving a spike-wheeled chariot or a hungry tiger, mammographers face dismal reimbursement rates, frequent litigation, a lack of enthusiasm among new radiologists, suspicious, noncompliant patients, and screening center closures despite the growing screening needs of an aging population. And let's not forget the mammography naysayers who are quick to criticize the efficacy of x-ray-based cancer screening, but are less forthcoming with alternative strategies.
So what's a cornered breast imager to do? To its credit, the community has come together to defend itself, whether by harnessing new technology, such as computer-aided detection, or assessing how established modalities (breast ultrasound, MRI, CT, scintimammography) can be used as weapons against breast cancer.
Of course, efforts have been made to tweak that old workhorse, film-screen mammography (FSM), most notably with the shift to full-field digital mammography (FFDM). While the latter modality is barely out of the gate (less than a decade on the market), breast imagers are not resting on their laurels: Soon to enter the arena is digital breast tomosynthesis (DBT).
Built upon FFDM technology, DBT combines 2D image slices to form a 3D composite exam. Trials indicate DBT images are superior to both FFDM and FSM in terms of quality and acquisition time. Early talk is that DBT not only will find breast cancers earlier, but also fix mammography's liability woes and restore its tarnished popularity among imaging specialists.
"DBT will eliminate diagnostic mammography," predicted Richard Moore, research director of the division of breast imaging at Massachusetts General Hospital (MGH) in Boston. "It's so much better, DBT makes (FSM) seem like a blunt instrument."
DBT basics
DBT utilizes conventional mammographic x-ray tubes and digital detectors, but the x-ray detectors swing over the breast in an arc, creating a series of image slices -- dense tissue is reduced to 1-mm thick slices -- that can be assembled and analyzed in multiple ways. More importantly, this parallax phenomenon helps to eliminate a problem that plagues FSM.
"DBT addresses one of mammography's important weaknesses, which is that of overlapping tissue," explained Joseph Lo, Ph.D. "It can obscure a lesion that is already there, because things are all pancaked together in a conventional mammogram. DBT can also help to better characterize lesions that you do see."
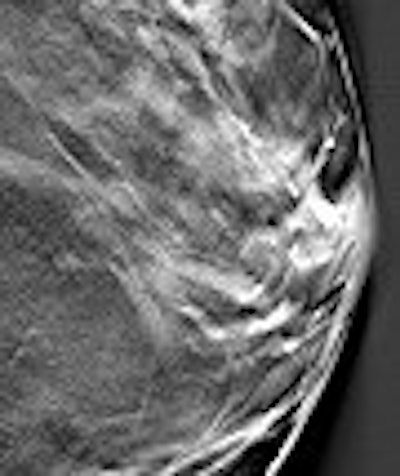
 |
| Above, conventional mammogram on which cancer is obscured. Below, DBT slice on which cancer is clearly defined. Images courtesy of Richard Moore and Dr. Daniel Kopans. |
 |
Lo is an assistant professor of radiology and biomedical engineering at Duke University Medical Center in Durham, NC, and is one of the developers of a DBT system by Siemens Medical Solutions of Malvern, PA.
Credited with producing clearer images than either FSM or FFDM, reading accuracy is improved and callbacks are reduced with DBT. The digital part makes for easier image storage/retrieval, and radiation dose is equal to or lower than that of conventional mammography, according to DBT proponents.
DBT is based on FFDM technology. Dr. Elizabeth Rafferty, Dr. Daniel Kopans, and colleagues at Massachusetts General Hospital began developing DBT technology in 1999, when FFDM first became available. They launched the first DBT trials in 2004 with a modified Senographe unit (GE Healthcare, Chalfont St. Giles, U.K.).
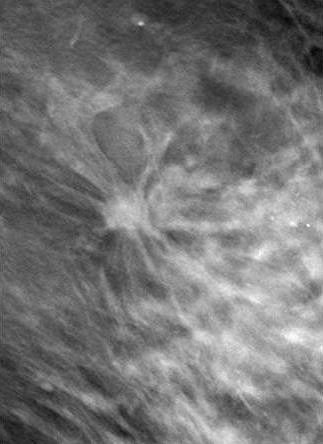
 |
| Patient who had previously undergone breast-reduction surgery. On left, surgical scars can be seen at the top half of DBT image. At right, tissue structures, which would have been partially obscured by the scar tissue, are visible. Images courtesy of Dexela and Mark B. Williams, Ph.D., University of Virginia, Charlottesville. |
Given that FFDM is a relatively new technology, why look to improve upon it so soon with DBT? As Moore pointed out, the idea of DBT has actually been around for some time.
"Dr. Kopans started working on 2D digital mammography in the late (1970s) with a scanning system made by American Science and Engineering," he said. "It took gorgeous images, but was way ahead of its time. In 1992, the National Digital Mammography Development Group (NDMDG) convened at our facility at MGH to academically collaborate on all aspects of digital mammography. At that time, our lab group felt pretty sure that DBT would pan out."
In 1997, the U.S. Army funded a pilot trial, Moore added. A year later, they funded a translational trial and GE Healthcare built the first prototype DBT scanner. The MGH group acquired 527 DBT studies on that machine from 2000 to 2004 but the scanner broke during a physical relocation. The next prototype, very close to a real product, was installed at MGH in June 2005, he said, and the group has done more than 2,600 DBT research mammograms to date. "The improvements are startling and obvious," he added.
Currently, four companies are developing DBT systems: Siemens Medical Solutions; GE Healthcare; Hologic/Lorad of Bedford, MA; and Dexela of London. While these systems are fundamentally similar, they differ from each other in terms of detectors, degree of x-ray arc, and number of slices per scan.
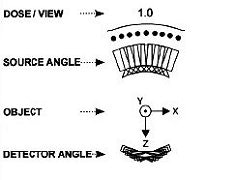 |
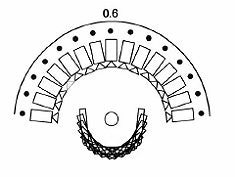 |
| Diagrams of the imaging geometry used in the Dexela DBT system. Top, narrow angular range limits 3D information. Above, regular angular spacing. Below, Dexela geometry with variable angular spacing, energy, and dose distribution. Images courtesy of Dexela. |
 |
The GE and Hologic systems vary in several aspects: The GE DBT unit obtains 15 slices in 12 to 23 seconds with a 40° arc. Hologic's obtains 11 slices over a 30° arc, but the company is not ready to disclose the scan time while it's still in the development stage, said Andrew Smith, Ph.D., Hologic's manager of imaging science.
Siemens won't divulge its OEM partners, but the company recently signed a deal to use Anrad detectors from Peabody, MA-based Analogic in all its FFDM systems. Siemens' system acquires 25 images over a 50° arc in about 13 seconds.
Finally, Dexela uses an experimental CCD-based digital detector and its DBT scanner can nab about 11 slices in 15 seconds over a 60° arc.
Out with the old, in with the new?
When the first FFDM system was brought to market in 2000, breast imaging experts, on the whole, proceeded with caution. Seven years later, FFDM systems are installed in about 15% of the mammography market. Cost has been one factor that may have caused people to hesitate in making the switch from FSM to FFDM: The latter unit goes for about $450,000 versus around $100,000 per unit for the former. A DBT system is expected to cost more than $700,000.
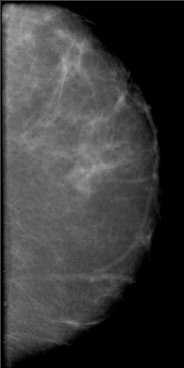 |
| A 2D mammogram generated from a tomosynthesis reconstruction using advanced iterative reconstruction. Image courtesy of Dexela. |
Another factor was the anticipation surrounding the Digital Mammographic Imaging Screening Trial (DMIST) results that were released in 2005. In brief, the researchers found that FFDM was a more accurate exam, but mostly in younger women and those with dense breasts. At the 2006 RSNA meeting in Chicago, DMIST lead investigator Dr. Etta Pisano and colleagues reiterated FFDM's strength in these particular patient populations.
FFDM's share of the North American mammography market jumped from 3% in 2003 to 13.8% in 2006, according to a report from market research and consulting firm, Frost & Sullivan of San Jose, CA ("North American X-Ray Mammography Markets," February 6, 2007).
In 2005, 11% of 37.7 million U.S. mammograms were performed with FFDM, according to a report by IMV Medical Information Division of Des Plaines, IL ("2005 Mammography Center Market Summary Report," March 22, 2006).
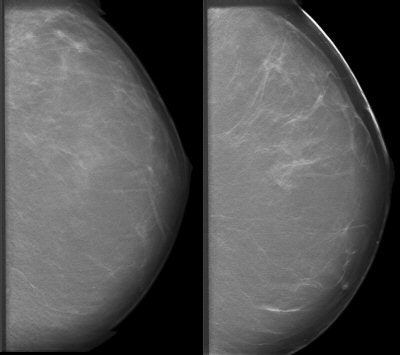
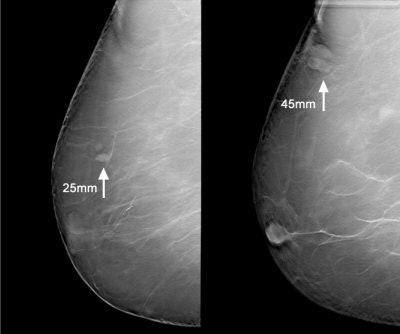
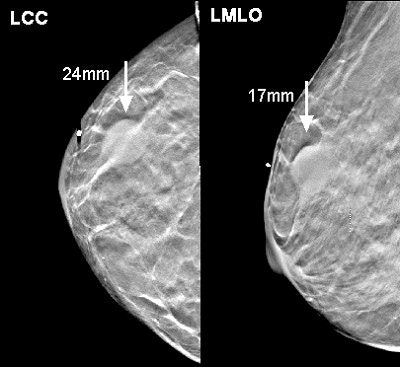 |
| Above, a DBT study in CC and MLO view with a dramatic lesion. Below, DBT study in CC view with two lesions at two different levels. Images courtesy of Dr. Laurie Fajardo, Breast Imaging Research, University of Iowa, Iowa City. |
 |
Developers feel DBT has the potential to far exceed FFDM's market share, if not replace FFDM and FSM altogether. "DBT was able to find a lot of lesions that conventional mammo was not," Lo noted. "We're really talking about replacing the role of mammography in mass screening for breast cancer."
"It does more, better," Moore said. "Conventional 2D digital mammography is demonstrated to be almost exactly the same as conventional 2D film-screen mammography. DBT is demonstrating better specificity -- much better -- and will very likely demonstrate better sensitivity."
Each DBT vendor claims its system is suitable for all breast imaging applications, including screening. Such an all-purpose solution theoretically eliminates a facility's need to own different systems for different applications, and therefore justify DBT's higher price tag.
DBT research
MGH's ongoing 3,000-patient trial has enrolled 2,400 cases so far, according to Moore. He said the group has seen the callback rate reduced by half, which could potentially mean 1.5 million callbacks per year in a clinical setting instead of 3 million with FSM.
Moore said the group has also seen the callback rate reduced by half, which could potentially mean 1.5 million callbacks per year in a clinical setting instead of 3 million with FSM.
This estimate is reinforced by a 2005 study conducted by Dr. Steven Poplack and colleagues at Dartmouth-Hitchcock Medical Center in Lebanon, NH. In that study of 100 recalled mammogram patients, the researchers found that half the callbacks would have been unnecessary had the original mammograms been made by DBT (Imaging Technology News, July/August 2006).
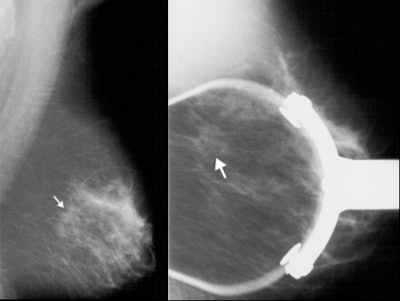 |
| Left, mammogram shows very subtle architectural distortion (arrow). Right, magnification views shows mass, which is still very subtle (arrow). Images courtesy of Joseph Lo, Ph.D., Duke University Medical Center, Durham, NC, and Siemens Medical Solutions. |
DBT also is expected to significantly reduce the number of biopsies needed. According to Smith, "some pathologies that are mammographically occult will be discernable through the elimination of structure noise. Noise reduction and tomosynthesis may allow improved detection of cancers in women with heterogeneously dense breasts" (Radiology Management, September/October 2005, pp. 25-31).
"Out of 80 (callbacks), we'll recommend 20 for biopsy, and four out of five will be negative," Moore said of their DBT experience.
In a session devoted to breast tomosynthesis at the 2006 RSNA meeting, several investigators, including those from MGH, discussed their experience with the modality.
First, Dr. Mark Helvie from the University of Michigan in Ann Arbor shared results from his group's study in which they compared tomosynthesis mammography to conventional mammography for lesion detection and reader preference.
Their patient population consisted of 11 breasts in 10 subjects (median age of 47) with abnormal mammograms or physical exams. Imaging was done on a prototype tomosynthesis/ultrasound system with a 60˚ arc, 21 projection views, seven seconds of compression, and 1-mm-thick slice reconstruction. The unit acquires 50 to 60 images per breast, Helvie said. Two experienced readers evaluated all images.
According to the results, 64% of the breasts were category III for breast density. The median lesion size was 13 mm and the majority was masses with one microcalcification case. The diagnoses included multiple cysts, fibroadenoma, and invasive ductal carcinoma.
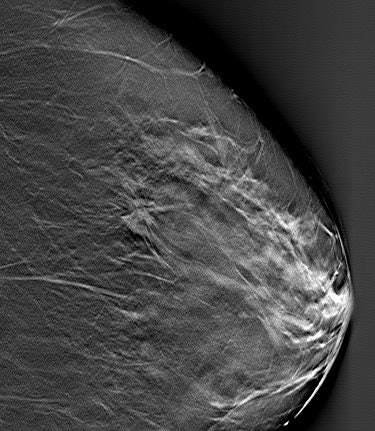 |
| DBT slice image revealed speculated mass (arrow). Biopsy revealed invasive cancer. Image courtesy of Joseph Lo, Ph.D., Duke University Medical Center, Durham, NC, and Siemens Medical Solutions. |
Based on tomosynthesis results, the two readers detected 28 lesions versus 21 lesions found on conventional mammography. There were four cancers found on eight images, with malignancies ranging in size from 1.5 cm to 2.5 cm. One reader did not see a mammographically occult 1.5 cm slightly irregular mass, but the other reader did, Helvie said. They recorded a 39.5% incremental increase in detection with tomosynthesis.
In addition, tomosynthesis was liked by the two readers, who gave the studies a mean score of 4.2 and 4.3 out of 5 ("strongly prefer tomosynthesis"). "Overall, each physician preferred tomosynthesis. We conclude that digital tomosynthesis mammography detected more masses than conventional mammography," Helvie stated. "Tomosynthesis detected more cancers than conventional mammography. Tomosynthesis was preferred by an experienced group of breast imagers for mass detection and characterization. We feel that tomosynthesis shows promise for screening for breast cancer."
In a subsequent talk, Moore elaborated on the topic of reader behavior with a study that compared reading for breast tomosynthesis to conventional mammography.
For this investigation, conventional mammography keystroke entry times for 10 experienced readers were culled in a database from December 2004 to March 2006. Digital breast tomosynthesis case readings, done during the first year of the National Institutes of Health and National Institute of Biomedical Imaging and BioEngineering (NIH-NIBIB) trial, were assessed by stopwatch.
According to the results for all 10 radiologists, conventional case reading time ranged from 15 to 270 seconds for data entry keystroke time in 55,181 readings on 28,262 women. Individual reading times varied from 40.7 seconds to 74.9 seconds. The mean time for BI-RADS 0 cases was 103 seconds, 45 seconds for BI-RADS 1, and 65 seconds for BI-RADS 2.
In the 547 tomosynthesis cases, the read time was a mean of 41.6 seconds (exclusive of data-entry time). The mean read time for BI-RADS 0 cases was 44.3 seconds, 41.3 for BI-RADS 1, and 40.5 for BI-RADS 2.
In a screening setting, the read time for tomosynthesis was comparable to conventional mammography for an acceptable level of workflow, Moore's group concluded.
In another study done at MGH, Rafferty and colleagues determined if breast lesions would be equally visible in the craniocaudal (CC) or mediolateral (MLO) view on breast tomosynthesis.
The patient population consisted of 34 patients who were scheduled for core or excisional biopsy. They underwent tomosynthesis of the involved breast in the CC and MLO positions. Data were acquired through 11 positions in a 28˚ arc during a 10-second sweep and reconstructed in 1-mm slices.
Direction comparison was made of the CC and MLO views for each index lesion, and lesion visibility was rated as equivalent on both acquisitions, more visible on one acquisition, only visible on one acquisition, or not visible on either CC or MLO views.
Of the 34 lesions, 16 were masses, seven were calcifications, three were masses with calcifications, and there were eight cases of architectural distortion. Sixty-five percent of the 34 lesions were equally visible on CC and MLO views while 12% were more visible on MLO, Rafferty reported. Fifteen percent were more visible on CC acquisitions and 9% were only visible on CC. All three of the latter lesions were malignant.
"Tomosynthesis renders mammographic lesions more visible by minimizing the impact of structural overlap," Rafferty stated. "In this pilot, 35% of the lesions were more visible or only visible on either MLO or CC tomosynthesis acquisitions. When performing best tomosynthesis, imaging both in MLO and CC is desirable to optimize visualization of lesions."
Rafferty added that because two views were needed for this study, radiation dose for the tomosynthesis exams was matched to that of a single conventional mammogram.
On the topic of radiation dose, Dr. Rüdiger Schulz-Wendtland from the University of Erlangen in Germany reported the following doses for their phantom experiments with a breast tomosynthesis prototype. Images of the phantom were acquired in 2D and tomosynthesis modes. Based on different anode/filter combinations, the estimated doses were as follows:
- Molybdenum-molybdenum (Mo-Mo): 2.1 mGy (63 mAs) for tomosynthesis
- Mo-Mo: 3.3 mGy (100 mAs) for conventional
- Tungsten-rhodium (W-Rh): 1.5 mGy (100 mAs) for tomosynthesis
- W-Rh: 1.9 mGy (140 mAs) for conventional
They found that they were able to successfully image all of the simulated structures in the phantom with tomosynthesis imaging for all filter/anode and dose combinations.
In a more recent study, researchers from the Hospital of the University of Pennsylvania in Philadelphia found that tomosynthesis offered a radiation dose comparable to that of a two-view mammogram. In their study, the dose of one tomosynthesis image (in a nine-image series) was approximately 20% of the dose of a single conventional mammographic view.
Dr. Sara Chen led her group in a pilot study that tracked their initial clinical experience with contrast-enhanced breast tomosynthesis. "The combination of contrast-enhanced digital mammography and digital breast tomosynthesis into a single technique would potentially integrate the benefits of both techniques, thus providing both breast cancer morphology and vascular information," they wrote (Academic Radiology, February 2007, Vol. 14:2, pp. 229-238).
For this research, 13 women were recruited to participate in the project, which was part of the National Cancer Institute's study on multimodality breast imaging. Images were acquired on an FFDM system (Senographe 2000D, GE Healthcare) in the MLO view only. The authors explained that more breast tissue could be visualized in this projection.
The breast was immobilized with 5-7 dN compression (versus 8-18 dN for a conventional mammography). Each precontrast tomosynthesis dataset consisted of nine images, acquired in 6.25˚ increments, over a 50˚ arc, at 49 kVp and 70-100 mAs with an Rh-Cu target/filter.
After a single dose of 1 mL/kg of contrast agent (Visipaque 320, GE Healthcare), nine additional tomosynthesis images were acquired. Pre- and postcontrast image sets were subtracted to optimize image display in stack mode on either a research workstation (eFilm v1.53, Merge Healthcare, Milwaukee, WI) or a GE workstation (Advantage Windows), the authors explained.
According to the results, 11 of 13 patients had pathologically proven malignancies, including ductal carcinoma and ductal carcinoma in situ (DCIS). Suspicious enhancing lesions were demonstrated on tomosynthesis images in 10 of the 11 cases.
"The precontrast and postcontrast tomosynthesis images provide localization of breast lesions in concordance with digital mammography," Chen's group concluded. "The precontrast tomosynthesis images also demonstrate lesion morphology and border characteristics."
Two studies out of Atlanta's Emory University evaluated radiation dose in DBT. They found that glandular dose was affected by compressed breast thickness, breast size, and the projection angle (Medical Physics, February 2007, Vol. 34:2, pp. 564-576; January 2007, Vol. 34:1, pp. 221-232).
Finally, the September issue of the American Journal of Roentgenology will feature a DBT paper by Poplack and colleagues that will discuss their experience with DBT in 98 women with abnormal digital screening mammograms (AJR, September 2007, Vol. 189:3).
DBT downsides
Whenever mammographic breast screening critics feel the need to point out that mammography is not a perfect test, they are, in essence, preaching to the choir: Mammographers are well aware that the modality is less than ideal, as are many other screening exams that are in widespread use today. There is no such thing as a perfect test and Baker readily acknowledged that this technology is no different.
For one, there is the potential for false-positive and false-negative studies. "With (DBT) we're going to see (benign) cysts and fibroadenomas more often," said Dr. Jay Baker, a radiologist at Duke and Lo's colleague. "I think we'll be willing to make the trade-off in exchange for finding far more worrisome breast cancer."
Then there is lack of compatibility between DBT systems and currently available biopsy devices. "The manufacturers need to develop a biopsy system for (DBT). Instead of having two views, you have 50," he explained. "We'll have to fall back on shooting a mammogram so we can see (the cancer) to do a stereotactic biopsy. It's just like MR -- there was no biopsy system for it early on, and you had to find things by ultrasound."
Another issue still to be resolved is how to manage electronic noise from the x-ray detector, which cause image degradation. A recent study of DBT simulation platforms by Jun Zhou and colleagues at the State University of New York reported that "there is a compromise between resolution, noise, and acquisition speed for a given glandular dose" (Medical Physics, March 2007, Vol. 34/3, pp. 1098-1109).
Time is another potential hurdle to overcome. "It's going to take longer to read these exams, particularly at first. Just getting through 50 images of a 5-cm breast -- particularly if you're doing four screening views, like we do now, that would be 200 images total -- is going to take more time. But if you spend more time on the screening and have fewer false-positives, you'll make up that time by calling back fewer people," Baker said. However, Moore disagrees about the time factor, noting that sharper images mean faster reading.
The new gold standard?
"(DBT) has the potential to replace mammography in pretty much all its current roles," including screening, said Lo, who reported on two of several DBT studies going on at Duke at the 2006 RSNA meeting.
Past and current clinical studies are designed to prove the science of DBT, but are they enough to make it the modality to cure the mammography blues? Does DBT hold tricks up its sleeve?
Yes, definitely, said its proponents. First, DBT offers plenty of bells and whistles that should attract the new generation of radiologists.
"Just the other day we found our first cancer that was only detected on the (DBT) scan. The subject wasn't there for anything other than her routine annual mammogram. When (DBT) becomes a clinical reality, we'll find these cancers that would've been missed by conventional mammography. Our residents are very excited about this technology," Lo said.
That excitement will hopefully offset some of the tedium that is inherent to mammography. "It's known for long hours: 'Here, read another 120 mammograms and enjoy yourself! It's not fun," Moore admitted.
Baker told AuntMinnie.com that residents and fellows at Duke "think (DBT) is the coolest thing.... It's a modality, like CT or MR, that they're used to looking at, and they like that. They're more interested in breast imaging already."
In fact, Frost & Sullivan credited DBT with "having the potential to significantly reverse the trend in facility closures and specialist flight exacerbated by the recent rise in mammography-related malpractice claims" ("North American X-Ray Mammography Markets," February 6, 2007).
Then there's the rather distasteful issue of litigation. No doubt mammographers are tired of hearing groups like the Physicians Insurers Association of America label the modality as the largest class of malpractice actions since 1995, with payouts to patients hovering near half a million dollars and based on the delayed diagnosis of breast cancer.
DBT's superior skills at zeroing in on cancers could make that "delay," real or otherwise, a moot point. "DBT might help us pick up cancers in dense breasts that we might otherwise have missed, or cancers that were subtle on a 2D image but in retrospect you could see them," said Dr. Laurie Fajardo, chair of the radiology department at the University of Iowa in Iowa City (Click here to view a video clip of a false-negative FSM case resolved by tomosynthesis).
"That's always the argument in medicolegal issues -- that someone didn't see it initially, but then a year or two later when you go back and look at that case, you can see a subtle change. (DBT) lets us see those better, and should improve the medicolegal aspects," added Fajardo, who is the leader of Hologic's DBT development team.
What about patient acceptance? The sheer discomfort of mammography can discourage compliance. While DBT doesn't require less compression, it does compress for a shorter length of time and every little bit helps. Lo said his group has done DBT exams on 215 patients to date and were pleasantly surprised to learn that the women found DBT more comfortable than conventional FSM. Another bonus for patients: Half as many callbacks means less women "worry(ing) about having cancer for two or three weeks until they can get (the report on) their images," Moore said.
Finally, there's reimbursement. Can DBT wow the folks at Medicare enough to make mammography financially viable once again?
Baker predicted that the DBT reimbursement course will follow the same path as FFDM, with the latter gaining greater acceptance once the DMIST results were released. "It took a big, definitive trial to say 'yes, it's better in this subset of points' to really open the floodgate," he said.
Moore said he believes the reduction of callbacks by half may be enough to sway government pooh-bahs in favor of DBT approval and reimbursement. "If you translate that nationally, that's a couple million studies per year that we don't have to do. So the regulators ought to love it."
Of course, DBT needs to make it to market before the benefits can start raining down. GE and Siemens, both of whom have DBT systems in final ongoing trials, said they may gain FDA marketing approval for their systems within the next two years. Hologic has finished its case collection for FDA approval and hopes to submit its reader trial in August. Dexela's system is still in phase I with early human clinical trials, according to Dr. Edward Bullard, CEO of Dexela.
"I would not be surprised to see DBT marketed and used in Europe and China in 2007, ahead of any U.S. marketing," Moore stated.
By Sydney Schuster
AuntMinnie.com contributing writer
June 21, 2007
Additional reporting by staff writer Shalmali Pal.
Disclosure notice: AuntMinnie.com is owned by IMV, Ltd.
Top image: "Pollice Verso" (1872) by Jean-Léon Gérôme.
Related Reading
British firm Dexela focuses on 3D mammography workstation, October 27, 2006
Expanded role looms for 3D in breast imaging, April 11, 2006
Residents leery of careers in breast imaging, December 15, 2003
Copyright © 2007 AuntMinnie.com



















