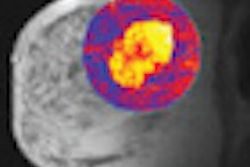
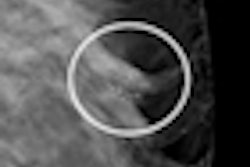
The U.S. Food and Drug Administration (FDA) will require Imaging Diagnostic Systems (IDSI) to file a premarket approval (PMA) application rather than a 510(k) for its CT laser mammography (CTLM) system.
The FDA characterized the CTLM technology as "diffuse optical tomography" (DOT), and said that it was not substantially equivalent to medical devices already on the market that are covered under the 510(k) program, such as CT or MRI scanners. As such, it is classified as a class III device and requires a PMA application.
IDSI said that while it was disappointed by the FDA's decision to require a PMA, the company will move forward with an application using data from international clinical trials it has conducted. The trials indicate that CTLM has higher sensitivity than x-ray mammography in finding breast cancer in women with extremely dense breast tissue, the firm said.