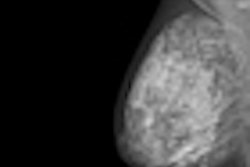
The U.S. Food and Drug Administration (FDA) has issued 510(k) clearance to Giotto USA for two versions of its full-field digital mammography (FFDM) technology.
The FDA cleared the company's Giotto Image 3D and 3DL systems, which feature a ring-shaped gantry that tilts to offer more flexible patient positioning. The 3D system features an 18 x 24-cm bucky, whereas the 3DL unit sports a larger 24 x 30-cm bucky.
Giotto USA is highlighting the Insight View image processing software on the system, as well as inclined patient positioning that is made possible by the tilting gantry. The unit's gantry can be slightly inclined, allowing the patient to be more relaxed and causing the breast to fall forward onto the bucky.
In addition, the units can also perform prone or upright stereotactic biopsy procedures with the company's Biopsy Digit device, giving users more flexibility.