CHICAGO - Philips Healthcare is highlighting its renewed push in mammography in its RSNA booth, as well as new product introductions in CT and the ongoing development of its PET/MRI program, which got a boost with a key regulatory clearance before the show. The company has also introduced new products in digital x-ray and ultrasound, as well as a new branding for its iSite PACS software line.
Mammography
Philips ramped up its footprint in the mammography segment in 2011 with its acquisition of Sectra's breast imaging business, and the company is showing the fruits of that deal in its RSNA booth with the MicroDose Mammography full-field digital mammography (FFDM) system, as well as the IntelliSpace breast workstation.
The company is highlighting the throughput possibilities with MicroDose Mammography, with some customers conducting up to 15 exams per hour. Philips also plans to promote the novel photon-counting digital technology used in the system, which the company believes reduces image noise.
The combination of a 50% dose reduction combined with a warm, padded, and breast-shaped contoured design is appealing to women, who want a more comfortable mammography exam appearance and are more radiation-dose aware, according to the company.
Philips began selling MicroDose Mammography in North America on October 31 and has several U.S. installations in place as it builds a dedicated women's health sales force. There are some 270 installations of the system outside the U.S., where it has been on the market longer as it awaited U.S. regulatory clearance.
Philips plans to continue selling its previous FFDM system, MammoDiagnost DR, which may be appropriate for sites that have a large volume of diagnostic work, while MicroDose Mammography handles screening studies.
CT
In CT, Philips is launching a new family of scanners called Ingenuity CT. Both systems are designed to enable Philips customers to easily upgrade to more powerful systems in the future.
 Ingenuity CT chest image processed with Philips' iDose4 iterative reconstruction algorithm at level 3 setting. Imaging parameters are 80 kVp, 91 mAs/slice, 32-cm coverage, 6.0-sec scan time, 1.7 mGy CTDIvol, 66.5 DLP (mGy-cm), and 0.9 mSv radiation dose.
Ingenuity CT chest image processed with Philips' iDose4 iterative reconstruction algorithm at level 3 setting. Imaging parameters are 80 kVp, 91 mAs/slice, 32-cm coverage, 6.0-sec scan time, 1.7 mGy CTDIvol, 66.5 DLP (mGy-cm), and 0.9 mSv radiation dose.
Ingenuity Core is an entry-level 64-slice scanner that features 4 cm of patient coverage. At the middle of the Ingenuity line is Ingenuity Core128, a 128-slice scanner with a 33% improvement in x-axis spatial resolution. At the top of the line is Ingenuity CT, which is also a 128-slice scanner, but which features additional technologies such as Philips' iDose4 iterative reconstruction dose reduction algorithm and O-MAR (orthopedic metal artifact reduction) algorithm.
Philips is highlighting the fact that scanners can be upgraded within the Ingenuity line easily and in the same scanning room, without the need for "forklift" upgrades. All systems have U.S. Food and Drug Administration (FDA) clearance, and will begin shipping in the fourth quarter. Philips executives said they would begin phasing out the company's older Brilliance CT line.
Philips is also highlighting clinical cases acquired with its iDose4 iterative reconstruction algorithm, which began shipping earlier this year. The studies include a chest case with an iDose4 level 3 image processing setting that resulted in 0.8 mSv of radiation dose at 80 kVp, and a case from China in which a brain study was acquired at a dose of 0.2 mSv. The company is touting a high-resolution inner ear case at a 1024 x 1024 matrix, which makes use of the ability of iDose4 to improve spatial resolution.
Philips announced that iDose would be migrated to its 16-channel scanners in late 2012, and it introduced faster reconstruction and throughput for the MX16 scanner.
In future developments, Philips is discussing research it is conducting on spectral imaging with dual-energy techniques, and the company also demonstrated a technique called iMR, which uses model-based reconstruction to create low-contrast detectability images that resemble MR scans. The technique is being shown as a work-in-progress and does not have FDA clearance.
Nuclear medicine
PET/MRI is being highlighted in the Philips booth, with the company shining a spotlight on its Ingenuity TF scanner, which features a 3-tesla MRI scanner integrated with a time-of-flight PET system. Ingenuity TF received 510(k) clearance for the system just prior to RSNA 2011.
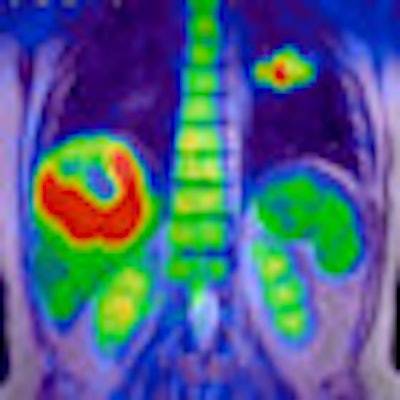
 PET/MR image acquired on Ingenuity TF indicates liver tumor.
PET/MR image acquired on Ingenuity TF indicates liver tumor.
Philips has received 15 orders for the scanner, five have been installed, and 500 cases have been acquired with the systems in all. At this year's RSNA meeting, Philips is announcing the ability to upgrade 3-tesla MRI scanners in its installed base to Ingenuity TF, a move that will enable the vendor to market upgrades to some 800 sites with 3-tesla MRI. Also new for RSNA 2011 is the ability to use the company's multitransmit coils on Ingenuity TF.
Philips is highlighting the radiation dose reductions possible by using MRI instead of CT as the anatomical modality with PET. In one case, a patient received only 13.6 mSv of total dose in the PET/MRI study, as opposed to 29.6 mSv from a PET/CT exam, in which the CT component contributed 16 mSv of dose.
In traditional nuclear medicine, the company is highlighting its penetration of the market with PET/CT systems based on time-of-flight (TOF) technology, with more than 300 TOF systems installed worldwide since the company introduced it in 2006. The technology leads to 30% better lesion detectability, significantly lower patient exposure, reduced tracer usage, and higher throughput. Some TOF sites are examining up to 20 patients a day, the company reports.
 |
| Ingenuity TF received 510(k) clearance just prior to RSNA 2011. |
At RSNA 2011, Philips is highlighting its TruFlight Select PET/CT scanner, first demonstrated at the 2011 Society of Nuclear Medicine (SNM) meeting. The company is touting the scanner's $1.4 million price point, which puts PET/CT technology within reach of even community hospitals and imaging centers, according to the company.
In SPECT/CT, the company is promoting the use of a flat-panel-based high-resolution coplanar CT module in the BrightView XCT hybrid system. This results in lower radiation exposure for both operators and patients, and the system fits in the same amount of space required for a conventional gamma camera. The system's coplanar geometry also has benefits for cardiac SPECT exams.
 Philips' TruFlight Select PET/CT scanner.
Philips' TruFlight Select PET/CT scanner.
On the workstation side, Philips is demonstrating its IntelliSpace portal available in the nuclear medicine section of its booth. The portal was introduced at the 2010 RSNA show and began shipping in July. The company is highlighting the efficiency improvements possible with use of the portal.
Philips has added a full suite of nuclear medicine applications to IntelliSpace, which previously mostly featured CT and some MRI tools. The portal also includes the ability to look at radiology studies outside those in nuclear medicine, enabling the possibility of visualizing images created via software fusion. Also supported are third-party cardiac and brain applications.
Digital x-ray
A wide range of new digital radiography (DR) and digital fluoroscopy products are being promoted in the x-ray section of the Philips booth at RSNA 2011.
DigitalDiagnost 3.0 is the newest version of Philips' high-performance DR system. The configuration being shown at RSNA 2011 has a wireless portable digital detector mounted on a multipurpose wall stand on rails. The wall stand is designed to move along the length of the table. The detector can be moved to a wireless tray in the table, thus accommodating all types of radiography examinations. DigitalDiagnost 3.0 has begun shipping.
Also in wireless DR, Philips is promoting its MobileDiagnost wDR, which was introduced at the RSNA 2010 meeting and began shipping in August. The high-performance model utilizes a 40-kW generator, which is ideal for both pediatric and bariatric patients.
 |
| Philips is featuring its DigitalDiagnost DR system. |
At this year's meeting, Philips has introduced a more economical version of its WPD wireless 14 x 17-inch digital detector to complement the premium version that was introduced last year. The company is promoting the fact that it now has a dozen wireless DR installations in place since the first unit was placed at a facility in Galway, Ireland.
Other new radiography developments include an extension of the FlexRoom suite, in a wall stand with multiple positions; a new version of the EasyDiagnost DR/Fluoro system; a new design for its EasyDiagnost 5.0 room with a new ceiling suspension, new display, and a new WPD detector tray; and finally, new capabilities for the EasyDiagnost workstation in its release 5 version.
Philips executives said that the wireless detector offers a more cost-effective way of increasing radiography exam capacity for customers. The mobility of the detector enables a customer to perform portable exams in the morning, and then transfer the detector to the EasyDiagnost room to perform fluoroscopy exams or handle rush overflow of radiography exams.
On the interventional side, Philips is introducing iXR, a hybrid operating room concept. The suite includes the Magnus patient table from Maquet, designed to meet the growing integration between catheterization labs and traditional surgery by providing a higher level of integration of the operating room table with the interventional x-ray system.
The suite also includes the company's FlexMove ceiling-mounted positioning system, designed to provide a flexible room layout, maximize patient access, and offer multiple parking systems for the C-arm.
Veradius Neo is a mobile C-arm with a flat-panel digital detector that Philips is launching at the RSNA 2011 meeting. The system was designed with input from surgeons, and includes features designed to improve its ease-of-use, such as color coding that indicates different movements and angles for better and more reproducible projections, according to the company.
The system also includes dose management features to reduce radiation dose during long minimally invasive procedures. Veradius Neo has not yet been cleared by the FDA and will be introduced in the summer of 2012.
 |
| Philips is launching its Veradius Neo mobile C-arm at the RSNA meeting. |
Finally, Philips is highlighting interventional oncology applications, such as image-guided therapy performed in the oncology suite. Interventional radiologists and surgeons can now make treatment decisions guided by instant feedback of needle advancement in 3D and in real-time, enabling them to plan and target drug delivery more precisely and avoid complications, according to the company.
MRI
In MRI, Philips is talking up clinical results from its Ingenia line of scanners, introduced at the 2010 RSNA meeting. The company began shipping the systems in July, and more than 100 are installed worldwide.
The Ingenia scanners feature a new technology that Philips calls digital broadband MRI, in which signals are digitized directly at the radiofrequency coil on the patient and are transmitted to the reconstruction engine via fiber-optic cable. The company claims that the technology results in a 40% increase in signal-to-noise ratio, and also results in a larger field-of-view.
Philips said that reports from users in the field indicate that digital broadband MRI is increasing the scan speed for routine applications like brain and musculoskeletal work. Users can also increase the resolution for exams without increasing scan times, and the technology's larger field-of-view also is a major benefit for oncology applications due to the need for soft-tissue resolution.
On the economic side, Ingenia users are seeing throughput improvements of 30% due to a reduction in coil handling. Because the technology is independent of the number of channels on a scanner, users can have lower upgrade costs when moving to applications in the future that require more channels. Philips has also reduced the installation time for a typical Ingenia scanner to seven working days.
Specific clinical applications that Philips is highlighting in its RSNA 2011 booth include whole-body 3-tesla oncology imaging, in which the technology detects lesions not seen on PET/CT. The company is also touting a C-spine procedure that took only six minutes of scan time.
Philips is also highlighting a work-in-progress software release for the Ingenia line, as well as support for new coils and new clinical functionality. Ingenia is available at 3-tesla and 1.5-tesla field strengths.
Outside the Ingenia line, Philips is highlighting enhancements in brain, body, and breast imaging for its Panorama HFO scanner, a superconducting 1-tesla open-bore system. The company is also showing an enhancement for the scanner that enables it to be used for radiation therapy and planning.
On the therapy side, the company is demonstrating an adaptation of its Sonalleve MR-HIFU high-frequency focused ultrasound system, designed to work with the Ingenia line. The adaptation is not yet cleared by the FDA and will be shown as a work-in-progress. The Sonalleve unit is designed for the treatment of uterine fibroids and adenomysosis, as well as the palliative treatment of bone metastases. The company has installed 25 systems worldwide, a number that includes commercial systems internationally and research units in the U.S.
Healthcare informatics
In PACS and imaging informatics, Philips is rebranding its iSite PACS software line in favor of IntelliSpace PACS. The new branding highlights the fact that PACS is part of a broader clinical informatics world within the healthcare enterprise, according to the company.
The current iteration of IntelliSpace PACS, version 4.4, includes productivity enhancements that reflect the growing reach of PACS outside the radiology department. Philips sees the company's goal with the software to help its users access information, become more efficient, and image-enable the electronic medical record or even create a new visual medical record that prominently features medical images. As a work-in-progress, it was demonstrated in a wide variety of mobile display devices.
IntelliSpace 4.4 includes workflow tools embedded in the software for improved collaboration and communication, the company said. Highlights include better image stacking, improved compliance with standards such as Integrating the Healthcare Enterprise, XDS, and XDS-I, as well as the ability to provide users with a single view of a patient's clinical history across multiple PACS applications.
The version includes support for clinical decision-making by bringing tools such as advanced visualization into the PACS application. Other enhancements include embedded mammography workflow that eliminates the need for a separate mammography workstation, a new viewer for cardiac studies that can be launched from the IntelliSpace platform, and a shared back-end infrastructure to support radiology and cardiology.
Finally, Philips is highlighting investments it has made in improving the infrastructure of the software, such as its ability to move customers to a virtualized software environment, which leads to an 80% reduction in server and electrical requirements.
Ultrasound
Philips is talking up its flagship iU22 ultrasound scanner, in particular the benefits of volume imaging applications performed on the system. The company is also promoting its xMatrix transducers, especially their ability to reduce sonographer wrist strain during scanning by reducing the number of orthogonal turns needed for abdominal exams.
A new transducer technology that Philips is promoting is its active-array technology, in which beamforming is performed in the transducer housing, a development enabled through the application of miniaturization technology. This supports digital beamforming while reducing the size of the cart needed for the scanner. Philips is demonstrating images acquired with the technology in vascular, ob/gyn, thyroid, and kidney applications.
ClearVue 500 is a new scanner designed as a multipurpose system for ob/gyn and shared-service markets. The scanner is designed to broaden the access to ultrasound into new clinical areas; it is pending FDA clearance.
Also, CX30 is a value-oriented scanner that creates a new price point under the firm's CX50 scanner. CX30 is a compact system, while ClearVue uses a traditional cart-based design, with one notable feature being the incorporation of part of the system's beamformer in each transducer.



















