Like it or not, the issue of breast density notification is here to stay, and women's imaging centers should educate themselves on how they will interact with patients and physicians on the issue -- whether or not their state has passed legislation, according to a presentation given on Sunday at the annual AHRA 2012 meeting.
For decades, women have accepted mammography as the gold standard for breast cancer screening. However, women are beginning to understand that their breast tissue density affects mammography's efficacy, and that radiologists and referring physicians often know their patients' breast tissue density but don't necessarily pass along the information, according to presenter Bonnie Rush, consultant and president of Breast Imaging Specialists of San Diego.
"We've been 'selling' mammography as the earliest form of detection for all women, but the truth is that breast density is one of the strongest reasons mammography fails to detect cancer," Rush said to AHRA 2012 attendees. "Women need to be informed to allow them to participate in personalizing their screening according to their risk profile."
What's currently required?
The Mammography Quality Standards Act (MQSA) does not mandate that breast density be mentioned in clinical reports, but the American College of Radiology (ACR) recommends that the information be included. In any case, notifying referring physicians of a woman's breast tissue density doesn't necessarily translate into patient notification, which boosts the risk of medical malpractice if the lack of communication leads to delayed diagnosis, Rush said.
States that currently have breast density notification mandates include Connecticut, New York, Texas, and Virginia. Meanwhile, California, Kansas, Maine, Missouri, Nebraska, New Hampshire, Tennessee, and Utah have endorsed notification bills, while Delaware, Michigan, Ohio, and Oregon have pending legislation.
But each of the laws differs slightly in its wording, according to Rush -- and the language doesn't necessarily promote further screening. What's needed is federal legislation.
"A national bill would put everyone on the same page," she said.
A federal bill is in the works: Introduced in October 2011, HR 3102, or the Breast Density and Mammography Reporting Act, is sponsored by Rep. Rosa DeLauro (D-CT) and has been referred to the House of Representatives' subcommittee on health. The legislation proposes that mammography results delivered to patients include language regarding specific breast cancer risk based on the breast density of each patient, as well as a statement that women with more breast density may benefit from supplemental screening tests. The bill also advises women to consult with their physicians.
"Unfortunately, there's no mention in the bill of how the supplemental tests will be covered," Rush said. "That creates issues about the economics of implementing any supplemental imaging strategies. Centers will have to determine if earlier detection has its own benefits -- such as the saving of women from late-stage diagnosis -- which could affect downstream revenues as those cancers are diagnosed and treated."
Legal concerns and false positives
The current cluster of notification bills emphasizes the importance of the patient-physician relationship for developing further strategies for early detection. But this could present a potential legal snare for the physician or the center, Rush said.
"Since these laws indicate the patient must take the initiative, the referring physician only gets involved if the patient contacts them and, therefore, is not responsible for tracking the patients who do not," Rush said. "Centers aren't mandated to track either. So what if the patient doesn't comply when supplemental screening is part of the notification?"
It may be advisable for the facility to track notified patients as a safety net if a lawsuit is brought for lack of communication that led to a delayed diagnosis, Rush said.
In addition, some women's health advocates are concerned that recommending that women be notified about breast density will lead to increased anxiety in the form of additional false positives with supplemental screening, Rush said.
"False positives and the 'anxiety' they produce is the most frequently cited objection to legislative efforts to communicate breast density and supplemental screening to women," she said. "But real anxiety comes with a late-stage diagnosis."
In fact, a survey conducted in 2011 by Are You Dense, a breast density notification advocacy group, showed that 93% of participants would elect to have additional screening if they were informed they had dense breast tissue, even if this additional screening produced false positives, Rush said. And nine out of 10 survey participants stated that a false-positive biopsy would not cause them to opt out of additional screening the following year.
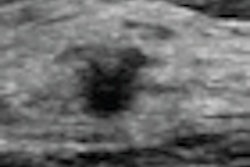
Determining breast density
But what's the best way to calculate density? One method is to use BI-RADS density scores, but these can differ based on who is reading the images, and risk assessment tools aren't yet accurate at profiling breast density versus cancer risk, Rush said. Using an automated system to calculate density could establish a more reliable metric, and, subsequently, breast cancer risk tools will catch up. These automated systems could also allow for accuracy in determining changes in density over time, so that risk assessment would be personalized.
There are two automated software packages on the market: Matakina International's Volpara and Hologic's Quantra. A technology called spectral mammography also holds promise for measuring breast density, as shown in a small study from the University of California, Irvine, Rush said.
Getting started
Centers need to extract breast density data as a first step to setting up a notification and education process for referring physicians, according to Rush. Then the center can start contacting patients.
"Data mining is easy with mammography reporting systems, while RIS-based mammography modules may not track density values, and users may have to go through each case manually," she said.
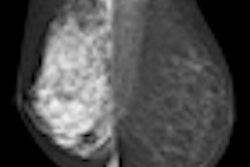
Centers also need to educate referring physicians about their dense breast imaging strategy so that these doctors can appropriately advise their patients, Rush said. Choosing a supplemental technology can be challenging, though. MRI, ultrasound, and digital breast tomosynthesis are all possibilities, but it can be unclear which is most effective.
MRI and ultrasound are mentioned in the existing notification laws. MRI has benefits for high-risk women, but it's costly and has a low compliance rate, Rush said. Studies show a twofold increase in cancer detection with ultrasound, but both MRI and ultrasound have high false-positive rates. Then there's digital breast tomosynthesis (DBT).
"DBT might do the job [as a supplemental modality for women with dense breast tissue]," Rush said. "It's been shown that it produces lower recalls than other technologies, and the preliminary data on the increase in cancer detection rate is positive. But more studies are needed to validate its benefits."
Changing landscape = opportunity?
In any case, heightened awareness about breast density and breast cancer risk has changed the women's health landscape. Because women direct 80 cents of every dollar spent on healthcare, centers should work proactively to reassure their patients and physicians that they are moving toward or have instituted a notification and personalized imaging strategy for these at-risk women, Rush said.
"Women and physicians will share the successes of your program," she concluded. "And since what consumers trust most is what others say, they will become your most effective marketing strategy."