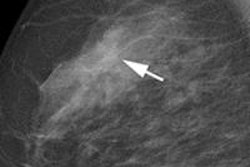
Computer-aided detection (CAD) developer VuCOMP has received U.S. Food and Drug Administration (FDA) premarket approval (PMA) of version 3 of its M-Vu CAD software for digital mammography.
This latest iteration of CAD technology drives the algorithm's false-positive rate down even further than version 2, according to the company. VuCOMP has just finished rolling out the new upgraded system to all M-Vu users at no additional charge.
M-Vu uses computer vision algorithms to identify areas of a mammogram that are consistent with breast cancer. Version 3 achieves 97% sensitivity for microcalcifications and 87% sensitivity for masses, while maintaining a total false-positive rate of 0.26 marks per image (one false positive per four-view study), VuCOMP said.
Version 3 of M-Vu is approved for digital mammography systems manufactured by a number of different vendors.