CHICAGO - At this week's RSNA 2015 meeting, Siemens Healthcare is unveiling a range of new imaging technologies, from a robotic x-ray room to a new entry-level PET/CT scanner. The firm is also talking up a regulatory clearance for CT lung cancer screening that it received just before the show opened.
X-ray/interventional
X-ray may be medical imaging's oldest modality, but Siemens is hoping to give the technology new legs with Multitom Rax, a new x-ray room that features a unique design: Both the 17 x 17-inch digital detector and x-ray tube are mounted in twin ceiling-suspended robotic columns. The design enables a level of automation and flexibility not previously found in conventional x-ray rooms, according to the company.
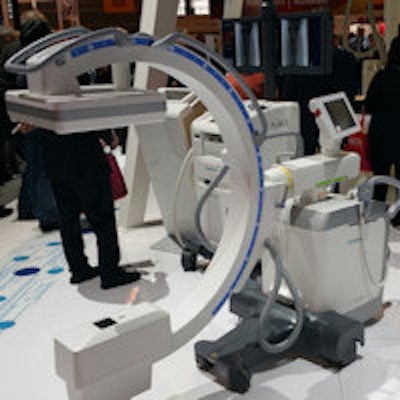
 Multitom Rax is a new x-ray room with twin robotic columns.
Multitom Rax is a new x-ray room with twin robotic columns.For example, Multitom Rax users can perform spins around knees and conduct weight-bearing studies, making full use of radiography's 0.24-mm spatial resolution -- superior to CT's 0.33-mm resolution.
The system supports classical radiography, 3D tomography, fluoroscopy, and angiography, and it can perform a wide range of clinical applications, from pediatric to bariatric imaging. It supports Siemens' Real 3D protocol, which is helpful for weight-bearing exams.
The company is also highlighting the automation built into Multitom Rax: Users can simply select an organ to be imaged and the system moves into position based on predefined presets. The company sees the system as a good fit for sites that do a lot of orthopedic work, or hospitals that want to consolidate several radiography and fluoroscopy rooms into a single room.
Multitom Rax was cleared by the U.S. Food and Drug Administration (FDA) last week, although Siemens is still waiting on the agency's nod for the 3D option. The system will begin shipping in April 2016.
In the mobile C-arm segment, Siemens is introducing three new midrange products in its Cios family: Cios Fusion, Cios Connect, and Cios Select.
Cios Fusion is available in 30 x 30-cm and 20 x 20-cm flat-panel digital detector sizes, and it also features software packages adapted from the company's flagship Cios Alpha C-arm, as well as an additional touchscreen that can be positioned at the operating table to control the C-arm from within the sterile work area. Cios Fusion is pending FDA clearance.
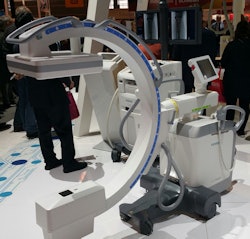 Cios Fusion is one of a new family of C-arms Siemens is launching.
Cios Fusion is one of a new family of C-arms Siemens is launching.Meanwhile, Cios Connect is a lightweight C-arm that weighs only 547 lb. It includes specialized software options that enable physicians to display individual images simultaneously in subtracted and x-ray views, for better presentation of contrast and bone. FDA clearance is pending on Cios Connect.
The third system in the family, the entry-level Cios Select, is designed to be a user-friendly option, with color coding to facilitate communication between surgeons and staff. Like other systems in the Cios family, Cios Select uses Siemens' intelligent dose efficiency algorithm (IDEAL) to provide continuous contrast and brightness adjustments as well as automatic dose-performance adjustment. FDA clearance is pending on Cios Select.
On the company's flagship Cios Alpha system, Siemens has released a software update that delivers a large preview image to enable staff in the operating room to select appropriate image settings more easily. A new one-touch metal correction function compensates for metal image components to enable the representation of surrounding tissue with greater contrast, according to the company.
In angiography, Siemens has added new features to its PURE suite of applications designed to simplify operations with its Artis angiography systems. Syngo EVAR Guidance offers better guidance for endovascular aortic repair (EVAR) procedures with automated detection of vessel walls on CT datasets and automatic placement of landmarks for 3D image guidance, while syngo CTO Guidance automatically segments coronary CT angiography (CTA) images.
Ultrasound
Siemens is highlighting ultrasound introductions made in recent months. First introduced at Medica 2015 earlier this month in Germany, Acuson NX3 and NX3 Elite are midrange systems suitable for general medicine, obstetrics/gynecology, pediatrics, and neurology applications, according to the vendor.
Key features include three times more customizable keys and 28% fewer keystrokes; a customizable control panel and touchscreen that enable the performance of routine anatomical measurements up to 76% faster than traditional approaches; and Siemens' Clarify Vascular Enhancement technology for tissue contrast resolution and definition of both tissue and vessel walls.
The systems are equipped with a 21.5-inch LED monitor and a 10.4-inch touchscreen, as well as a 16-MHz transducer with particular benefit in breast and musculoskeletal imaging. A 220° endocavity transducer offers up to a 75% larger field-of-view compared with standard probes, Siemens said.
The NX3 Elite edition adds features such as an adjustable control panel, advanced transducers, and the firm's eSie Touch elastogram technology.
Siemens is also pointing to its HELX Evolution with Touch Control hardware and software redesign for its Acuson S family of ultrasound scanners. The redesign, which was announced in September, brought a new user interface aimed at making it simpler for users to operate the S1000, S2000, and S3000. For example, Siemens has decreased the number of software keys by 44% and the number of tactile keys by 33% compared with previous versions. In addition, there are 22% fewer home base controls.
The vendor is also emphasizing the new touch display; new imaging optimization technologies for abdominal, breast, vascular, and musculoskeletal exams; and improved shear-wave elastography techniques added as part of the upgrade.
Molecular imaging
Biograph Horizon is the highlight in the molecular imaging area of the Siemens booth. First shown at the American Society for Radiation Oncology (ASTRO) meeting in San Antonio in October, Horizon is designed for the entry-level hybrid imaging segment.
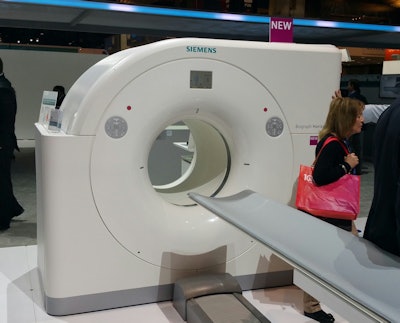 Biograph Horizon is an entry-level PET/CT scanner.
Biograph Horizon is an entry-level PET/CT scanner.While Horizon features the same PET module used on Siemens' high-end PET/CT scanner, with 4-mm lutetium oxyorthosilicate crystals, it employs a 16-channel CT component based on the company's Somatom Perspective to keep costs lower. Siemens also focused on reducing the total cost of ownership with the system, and designed it with a smaller footprint to make it easier to site.
Horizon can perform time-of-flight (TOF) studies and also has protocols for metal artifact reduction and dual-energy image acquisition. FDA 510(k) clearance is pending for the system, with shipments expected to begin in April 2016.
CT
The big news in the CT section of Siemens' booth is the company's receipt of FDA clearance to market its scanners for CT lung cancer screening. The clearance applies across the company's Somatom CT product line.
Siemens is in the process of communicating to its customer base about the clearance; at the RSNA meeting, it is showing a Somatom Scope scanner in a mobile van as an example of how lung screening can be brought out to the community in an effort to improve screening compliance rates.
Siemens is also marking the 10th anniversary since the launch of its first dual-source CT scanner.
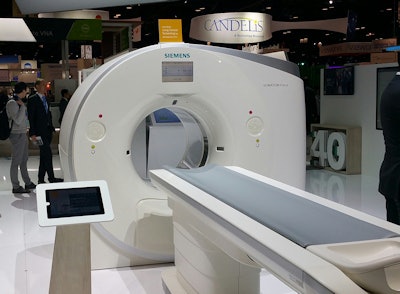 Siemens has received FDA clearance for CT lung cancer screening on all of its CT scanners, including the Somatom Force shown here.
Siemens has received FDA clearance for CT lung cancer screening on all of its CT scanners, including the Somatom Force shown here.Informatics
In the informatics arena, Siemens is highlighting Protocols, a new imaging protocol management function on its Teamplay collaboration platform. With the new module, users can view and compare protocols on Siemens CT and MRI scanners. Protocols can be sent to imaging modalities to facilitate standardization, according to the vendor. The new offering will be available in early 2016.
The firm is also showcasing version VB20 of its syngo.plaza radiology enterprise management software. The release includes modules for reading and reporting, viewing, and archiving, Siemens said.
A new user interface features enhancements such as a "calmer" button style, a streamlined area for image navigation functions, and an optimized color scheme to help radiologists focus on relevant image information. The software can also automatically scale the user interface to fit the resolution of the device being used to review the imaging studies. Siemens said it has also added new synchronization features to improve comparison of images in the same study as well as with previous exams.
In advanced visualization developments for syngo.plaza, Siemens has upgraded its 3D+ option to include the firm's automatic landmarking and parsing of human anatomy (ALPHA) technology. ALPHA features such as AutoViews and anatomical range presets automate and standardize 3D reconstructions and views, yielding workflow enhancements and improved consistency for reconstructing and presenting images, according to the company.
 Siemens is emphasizing its digital breast tomosynthesis technology at McCormick Place.
Siemens is emphasizing its digital breast tomosynthesis technology at McCormick Place.Syngo.plaza VB20 also supports dedicated archiving packages as well as syngo.share, accommodating radiological vendor-neutral archiving of DICOM studies and enterprise-wide multimedia vendor-neutral archives that can consolidate archiving of all data from clinical and radiological departments into a central repository.
MRI
In MRI, Siemens is rolling out new clinical applications for its Prisma scanner that were first seen on the Magnetom Skyra and Aera scanners. Simultaneous multislice (SMS) is a new protocol for acquiring MRI data with multiple slices at once, providing faster data acquisition. It's currently available for neuro applications but will also be rolled out to other areas of the body.
Another new application, GoBrain, was developed in collaboration with Massachusetts General Hospital. It is designed to allow brain studies to be performed more quickly, in as few as five minutes. FDA clearance is pending for both applications.
Women's imaging
After becoming the third vendor to receive FDA approval for digital breast tomosynthesis (DBT) technology back in April, Siemens is shining the spotlight on a DBT add-on option for its Mammomat Inspiration digital mammography platform.
Siemens has 64 customers in the U.S. using the DBT system, along with a significant backlog, according to the firm.