The American College of Radiology (ACR), Breast Cancer Research Foundation (BCRF), and GE HealthCare are enrolling the first patients in the Contrast-Enhanced Mammography Imaging Screening Trial (CMIST).
The study aims to find out whether contrast-enhanced mammography (CEM) helps with breast cancer detection and reduction false-positive exams in women with dense breasts. Standard mammography struggles with imaging dense breasts, meaning breast density is a risk factor in breast cancer development.
The U.S. Food and Drug Administration (FDA) recently announced a change in the Mammography Quality Standards Act (MQSA) that will require patient notification about their breast density.
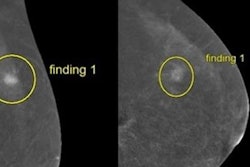
CEM adds iodinated contrast media to mammography, highlighting blood flow patterns in breast tissue. The researchers aim to enroll a total of 2,032 women with dense breasts in CMIST, comparing CEM to 3D mammography.
The group also indicated that 15 sites in the U.S. and Canada are planned to start recruiting patients, with Carolina Breast Imaging Specialists in Greenville, North Carolina being the first. The team said it plans to publish the first results in 2025.