Orthopedic digital imaging firm EOS Imaging has received U.S. Food and Drug Administration (FDA) 510(k) clearance for version 1.5 of its sterEOS 3D imaging software.
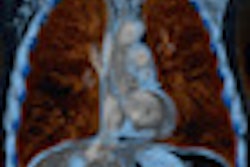
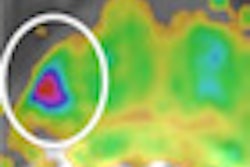
The new software version adds expanded calculation and analysis functionality, with new capabilities to measure hip implant component position for surgery control and revision, the company said. The vendor has also incorporated 3D modeling of severe scoliosis.
EOS is highlighting the software along with its EOS digital radiography (DR) system at this week's American Academy of Orthopaedic Surgeons (AAOS) annual meeting in Chicago. The new release will be available to existing customers as a software upgrade and will be included in all new system sales, EOS said.