A software-based "virtual heart" created with data from cardiac MRI scans could be a better tool for predicting which patients should receive implantable cardioverter defibrillators (ICDs). In a proof-of-concept study, researchers found that the heart model outperformed other metrics for predicting future arrhythmias.
ICDs can be a lifesaving technology for individuals with heart arrhythmias, delivering electric pulses that can correct irregular heartbeats, restore normal heart rhythm, and prevent sudden cardiac death. But many patients receive ICDs who don't need them, according to researchers from Johns Hopkins University. This exposes patients to infections, device malfunctions, and inappropriate shocks, they wrote in an article published May 10 in Nature Communications.
There are already existing metrics for determining risk of sudden cardiac death, one of which is left ventricular ejection fraction (LVEF): An LVEF of less than 35% has been considered the threshold for high risk.
But LVEF is a crude tool that fails to apply to most victims of cardiac death; as a result, only about 5% of ICD placements are believed to be appropriate, wrote a team led by Hermenegild Arevalo, a postdoctoral fellow of the Institute for Computational Medicine and the department of biomedical engineering at Johns Hopkins.
What if you could create a computer model of a patient's heart to determine whether the person is susceptible to arrhythmia and, thus, should get an ICD? They decided to test this theory in a small group of 41 patients with histories of prior myocardial infarction.
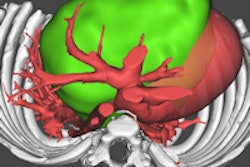
The researchers developed an approach they call the virtual-heart arrhythmia risk predictor (VARP). First, data from contrast-enhanced MRI scans are used to construct 3D ventricular wall geometry for a specific patient, and pixels in the model are classified as infarcted or noninfarcted based on their signal intensity. Infarcted tissue can further be classified as scar or gray zone, a sort of border area that can contribute to arrhythmia.
When the VARP model has been completed, electrophysiological properties are assigned to the model elements -- for example, areas of scar tissue are considered to be electrically nonconductive. Then, the model is electrically stimulated via pacing from 19 different locations in an effort to elicit arrhythmia. The patient is considered at risk for sudden cardiac death if an arrhythmia is produced from one of the locations.
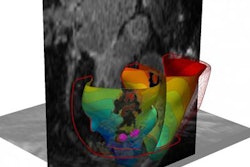
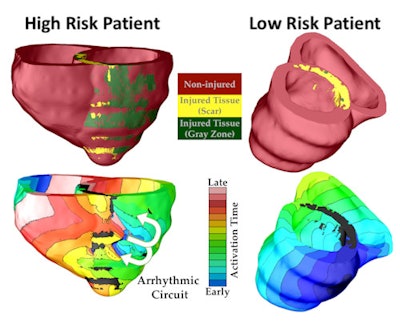 The virtual heart methodology classified one patient as high risk of developing a life-threatening arrhythmia, and the other as low risk. Top panels show the geometric replicas of the patient hearts, reconstructed from their MRI scans. Shown is normal tissue as well as injured tissue left by an earlier heart attack in each heart. The injured tissue consists of scar and a semiviable border zone between normal tissue and scar (also called gray zone because of its gray appearance in the MRI scan). Bottom panels show propagation of electrical wave in the virtual hearts, with lines representing times at which the electrical wave arrived at given locations in the heart (called activation times). In the low-risk heart, despite the presence of injury, the electrical wave swept uniformly through the heart, triggering strong uniform contraction and blood pumping. In the high-risk heart, arrhythmia developed. The electrical wave was "stuck" at the scar, rotating around it over and over again, which is called arrhythmia (arrows show direction of rotation, i.e., the arrhythmia "circuit"). The rotational wave was unable to cause coordinated contraction and prevented the heart form pumping blood effectively, which results in sudden cardiac death unless a defibrillation shock is given. Image courtesy of Hermenegild Arevalo and Natalia Trayanova.
The virtual heart methodology classified one patient as high risk of developing a life-threatening arrhythmia, and the other as low risk. Top panels show the geometric replicas of the patient hearts, reconstructed from their MRI scans. Shown is normal tissue as well as injured tissue left by an earlier heart attack in each heart. The injured tissue consists of scar and a semiviable border zone between normal tissue and scar (also called gray zone because of its gray appearance in the MRI scan). Bottom panels show propagation of electrical wave in the virtual hearts, with lines representing times at which the electrical wave arrived at given locations in the heart (called activation times). In the low-risk heart, despite the presence of injury, the electrical wave swept uniformly through the heart, triggering strong uniform contraction and blood pumping. In the high-risk heart, arrhythmia developed. The electrical wave was "stuck" at the scar, rotating around it over and over again, which is called arrhythmia (arrows show direction of rotation, i.e., the arrhythmia "circuit"). The rotational wave was unable to cause coordinated contraction and prevented the heart form pumping blood effectively, which results in sudden cardiac death unless a defibrillation shock is given. Image courtesy of Hermenegild Arevalo and Natalia Trayanova.To test the VARP concept, the researchers employed it retrospectively with 41 patients who had a prior history of myocardial infarction and left ventricular ejection fraction below the 35% threshold. All patients had received ICD implants and underwent MRI scans prior to implantation; 21 had arrhythmic events and 20 did not. Patients were followed for an average of 4.8 years.
The researchers found that if an arrhythmia was induced in a patient's VARP model, that patient was four times more likely than a patient who had a negative VARP test to be at risk of the study's end point -- defined as the ICD firing due to ventricular arrhythmia or cardiac death. The VARP test was also more accurate compared to other clinical factors, as indicated in the table below; only the VARP model had a statistically significant association with ICD firing.
| Accuracy of risk models for predicting ICD firing | |||||
| Risk model | Hazard ratio for arrhythmia | p-value | |||
| VARP | 4.05 | 0.03 | |||
| LVEF | 0.95 | 0.12 | |||
| Gray zone volume | 1.02 | 0.26 | |||
| Scar volume | 1.02 | 0.16 | |||
| Left ventricular mass | 1.00 | 0.98 | |||
When appropriate (as oppose to inappropriate) ICD firing was used as an end point, the VARP model's predictive prowess improved even more, to a hazard ratio of 5.0.
The researchers concluded by stating that the VARP model would prevent many unnecessary ICD implant procedures and their associated complications. The model could even be expanded to patients who have less risk of sudden cardiac death than those in the current study, such as those with LVEF higher than the 35% threshold; these patients are not targeted for therapy under current guidelines.
The authors concluded by highlighting the potential of the VARP model in cardiac medicine.
"This study makes a leap forward in integrating image-based computational modeling of the heart into heart disease diagnosis and treatment," the group concluded. "We believe that computer modeling is poised to transform areas of medicine and serve as a vehicle to advance personalized approaches to human health."