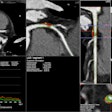
Augmented reality company Body Vision Medical has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market LungVision, an imaging software that enables real-time navigation and lesion localization during bronchoscopic procedures.
LungVision uses augmented reality so physicians can plan, visualize, and track endobronchial tools and radiolucent lesions in real time. LungVision employs intraoperative fluoroscopy with preoperative high-resolution imaging, such as CT.