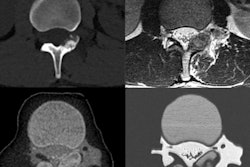
The U.S. Food and Drug Administration (FDA) on December 4 released a new set of recommendations for creating medical products using 3D printers. The guidance details the agency's stance on device design, testing, and quality system requirements.
For a relatively new technology, 3D printing has a wide range of clinical applications, such as building functionally accurate replicas of complex anatomical structures and facilitating surgery simulations to restore hearing loss, remove stroke clots, and repair hip disorders. The rapid evolution of this technique has led the FDA to release an early policy framework to help manufacturers bring 3D-printed models to the market more efficiently.
"The FDA is now preparing for a significant wave of new technologies that are nearly certain to transform medical practice," FDA Commissioner Dr. Scott Gottlieb said in a statement. "We're working to provide a more comprehensive regulatory pathway that keeps pace with those advances and helps facilitate efficient access to safe and effective innovations that are based on these technologies."
Developed in part based on the FDA's August 31 joint workshop with the RSNA's 3D Printing Special Interest Group, this new guidance focuses on the technical aspects of 3D printing that the agency recommends manufacturers report on submission for 3D-printed medical devices.
The technical considerations are divided into separate topics:
- Design and manufacturing process. This section covers technical aspects of 3D-printed models that should be addressed to satisfy quality system requirements. With respect to imaging, manufacturers should take into account several factors when creating patient-specific models with imaging data such as CT scans: minimum image quality and resolution, smoothing or image processing algorithms that may alter the dimensions of the model compared to patient anatomy, the rigidity of the anatomy being imaged, and the clarity of anatomic landmarks used to tailor the model.
- Device testing. Device description, mechanical testing, dimensional measurements, material characterization, cleaning and sterilization, and biocompatibility are important features for premarket submission of 3D-printed devices.
- Labeling. Each device should have labeling that includes a patient identifier, device use and final design, and a precaution to survey patients for any bodily changes before applying the device.
This "leapfrog" guidance presents the agency's initial thoughts on the advancing technology -- notions that are likely to progress along with advances in 3D printing, Gottlieb said.
"Innovative developments in the technology are already emerging at hospitals and academic centers for use in clinical studies," he said.
The next step for the FDA is to develop a policy on 3D printing that explores the role of nontraditional manufacturing facilities such as hospitals and university labs and to review the regulatory issues tied to the bioprinting of biological, cellular, and tissue-based products.
"3D printing is certain to alter the daily practice of medicine where patients will be treated with medical products manufactured specifically for them," Gottlieb said. "The FDA has an important mission to help advance these efforts while also protecting patients who depend on medical products to be safe and effective."