Dear AuntMinnie Member,
The U.S. Food and Drug Administration (FDA) yesterday released a new guidance on its regulatory approach to 3D printing in medicine. The guidance should give some clarification to both developers and users of 3D-printed products for clinical applications.
The field of 3D printing has been burgeoning as clinicians explore the technology for customized implants, education, and other uses. However, the regulatory landscape has been something like the wild, Wild West.
The FDA's new guidance explains the agency's stance on device design, testing, and quality system requirements, and it also includes a special section on the use of medical imaging to create 3D models. Learn more by clicking here.
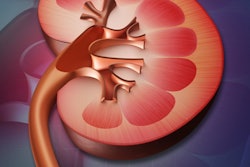
In other 3D printing news, researchers in Germany created low-cost, 3D-printed kidney phantoms for quantification of SPECT/CT studies. Find out how well they worked by clicking here, or visit our Advanced Visualization Community at av.auntminnie.com.
New MRI safety app
Most of the discussion about MRI safety recently has focused on gadolinium deposition, but managing the powerful magnetic fields of an MRI scanner remains an important consideration. What if you had an app that could tell you where in the MRI suite the magnetic fields are strongest?
Fortunately, such an app now exists, thanks to MRI safety expert Dr. Emanuel Kanal of the University of Pittsburgh. It's called MagnetVision, and we interviewed Dr. Kanal about it at last week's RSNA 2017 meeting. Click here to learn more.
If you haven't had a chance to check out the rest of our coverage of last week's conference, be sure to visit our RADCast @ RSNA special section, at radcast.auntminnie.com.
CCTA discrepancies
Finally, another interesting study from RSNA 2017 discovered discrepancies between core labs and local facilities in the way coronary CT angiography (CCTA) studies were interpreted. Learn more by clicking here.