Dear AuntMinnie Member,
Are you intrigued by the potential of 3D printing for clinical applications but unsure how to get started? Well, you'll want to read the new guide to 3D printing we've just published in our Advanced Visualization Community.
Presented in two parts, part 1 of the guide covers many of the big questions about 3D printing, ranging from start-up costs to convincing hospital administrators to commit the funds needed to set up a 3D printing lab. One piece of advice: It's better to start small with a simple project than be overly ambitious. Read more by clicking here.
Part 2 covers how to capture the value of 3D printing -- namely, quantifying the dollars saved by reducing operating time or costs, as well as reduced training and other intangible benefits. Get the rest of the story by clicking here, or visit the community at av.auntminnie.com.
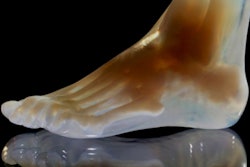
Before you leave the community, make sure you check out this story on a 3D printing technique that makes it easier to convert data from CT and MRI scans into complex anatomical models.
News and videos from SIIM
The Society for Imaging Informatics in Medicine (SIIM) meeting wrapped up over the weekend in National Harbor, MD, and Senior Editor Erik L. Ridley was on hand, providing tweets, articles, and video reports with major luminaries in imaging informatics.
For example, check out this article on the use of an artificial intelligence (AI) algorithm to detect microbleeds on brain MRI scans, or this story on how AI fits into the value-based healthcare system, based on a talk at the Data Science Summit that preceded SIIM 2018.
Also be sure to check out this video interview with Dr. Curtis Langlotz, PhD, on the major trends in AI and radiology, as well as this interview with Dr. Bibb Allen Jr. Finally, don't forget this discussion with Paul Nagy, PhD, about the major issues in informatics being discussed at SIIM 2018, and this chat with Dr. Tessa Cook, PhD, on efforts to increase career opportunities for women in radiology and imaging informatics.
FDA to ease CAD regulations
In other news, the U.S. Food and Drug Administration (FDA) on Friday announced a proposal to change the way it regulates computer-aided detection (CAD). The FDA said it would downgrade CAD and other types of image analysis software from class III to class II medical devices, which means they would be able to go through a less rigorous regulatory process in order to get to market. Learn more by clicking here, or visit our Artificial Intelligence Community at ai.auntminnie.com.