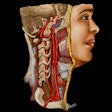
The American College of Radiology (ACR) has recommended several changes to a discussion paper issued by the U.S. Food and Drug Administration (FDA) in December on the use of 3D-printed medical devices.
In a February 7 letter to the FDA, ACR Chair Dr. Howard Fleishon said that for 3D printing activities relating to radiology, anatomic models created by healthcare facilities are appropriately characterized as meeting the FDA's "very low risk" designation. Accordingly, the ACR is recommending that the FDA exercise enforcement discretion for 3D-printed anatomic models and other very-low-risk devices created by the same healthcare facility that's responsible for the medical use of the device.
"This targeted approach to exercising enforcement discretion for lower-risk devices would be consistent with how FDA has approached low-risk subtypes of software device functions in certain areas of digital health oversight, such as clinical decision support, mobile medical applications, and medical device data systems," Fleishon wrote.
The ACR is also recommending that the FDA use the term "healthcare facility" instead of "point of care" for describing the setting for 3D printing, as the group noted that 3D printers are not typically located in patient procedure rooms.
In addition, the healthcare experts and components involved in the 3D printing process may not always be physically located in the same buildings or areas, according to the ACR.
"Moreover, a [healthcare facility] performing the printing could serve in a traditional manufacturer-type capacity to a disparate [healthcare facility] where the patient care is provided using the 3D-printed device," Fleishon wrote. "Therefore, some delineation between [healthcare facilities] that create the device and [healthcare facilities] that use the device may be necessary if separate entities, but without using '[point of care]' as the descriptor."