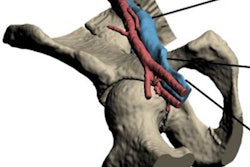
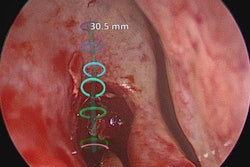
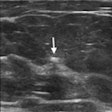
The U.S. Food and Drug Administration (FDA) has cleared augmented reality company Body Vision Medical's LungVision lung navigation catheter, used with bronchoscopes and the company's LungVision system to guide endotherapy tools to small pulmonary nodules.
The agency cleared Body Vision Medical's LungVision imaging and navigation system in May 2017. Since then, the system has been used in more than 290 clinical procedures in 10 lung cancer centers across the U.S. The company said it plans to present clinical results from a multicenter study with the system at the upcoming American Thoracic Society (ATS) meeting in San Diego.