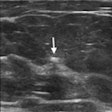
The U.S. Food and Drug Administration (FDA) has granted breakthrough device designation to IceCure Medical's ProSense liquid nitrogen-based cryoablation system.
The designation covers the use of the system for treatment of patients with T1 invasive breast cancer and accelerates the FDA's clearance process for this indication, according to the firm. The device is already FDA-cleared for general minimally invasive cryoablation applications, including for kidney, liver, and benign breast tumors, IceCure said.