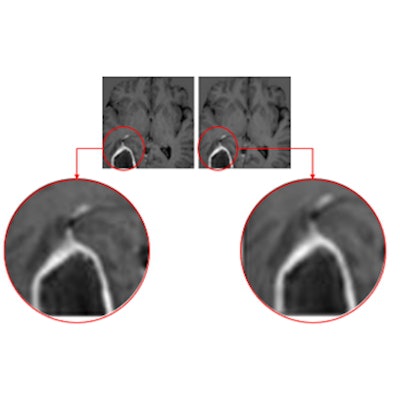
Advanced visualization software developer Imaging Biometrics has submitted a 510(k) application to the U.S. Food and Drug Administration (FDA) for its IB Zero G artificial intelligence-based software application.
IB Zero G is designed to eliminate the need for gadolinium-based contrast agents (GBCAs) in routine MRI exams, according to the vendor. The company received a patent in 2021 for the software.