VIENNA - Philips Healthcare is highlighting its expanding prowess in data analytics at ECR 2016 this week, as well as additional upgrades and enhancements across its product portfolio.
Philips is leveraging the technology and expertise in data analytics developed over the years in its equipment service operation with the HealthSuite digital platform (HSDP), moving it forward to enable customers to analyze their own operations. The company is now helping sites analyze and interpret data in a way designed to prevent problems before they develop.
Philips is in the process of enabling its customer sites to use the cloud-based HSDP network, which for years has been storing customer data that the company uses for its maintenance and service accounts. The vendor is in the process of "productizing" the network, according to Robert Cascella, CEO of the company's Imaging Systems business group.
In the realm of big iron, Philips is promoting its broad range of products in its booth at ECR 2016. The company has made a major priority of simplifying its product line to make its systems easier to use, Cascella said.
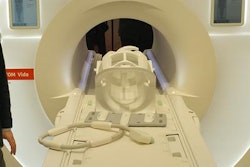
In MRI, the company is highlighting ScanWise Implant, an automated guided user interface for selecting the best scanning sequence for patients with MRI-conditional implants. Users simply enter the type of implant a patient has along with other patient characteristics, and the most appropriate scan sequence is selected. ScanWise Implant is initially available on Philips 1.5-tesla scanners such as the Ingenia 1.5T S, and it is not yet available in the U.S., pending regulatory clearance.
 Philips is showing the ScanWise Implant protocol optimization feature for MRI scanners such as the Ingenia 1.5T S, shown here.
Philips is showing the ScanWise Implant protocol optimization feature for MRI scanners such as the Ingenia 1.5T S, shown here.Also in MRI, Philips is highlighting its MR In-Bore Patient Experience, designed to help patients cope with claustrophobia during scans with a video display monitor outside of the scanner that can show soothing content like nature scenes. Philips recently added the ability to display patient instructions on the monitor.
In addition, mDixon XD is an MRI sequence for fat-free imaging that is designed to improve sharpness and help radiologists visualize abnormalities that might otherwise be obscured by fat, serving up two contrasts in a single scan.
In CT, Philips is emphasizing its IQon spectral CT system, and a new Spectral Magic Glass on PACS mode that enables users to review and analyze spectral data retrospectively on the PACS. Philips' goal with IQon is to allow users to perform spectral CT without having to change their workflow. The company is taking orders for IQon, and the system will begin shipping in the second quarter.
Other Philips highlights include its ultrasound portfolio with the Epiq, Affiniti, ClearVue 850, and CX50 ultrasound scanners. The company's Lumify transducer, which plugs into a smart mobile device to enable app-based ultrasound, is being shown as a work-in-progress.
The latest release of the company's IntelliSpace Portal 8.0 software is also being shown, which includes advanced data sharing, analytics, and visualization. Introduced at RSNA 2015, the platform offers applications to manage workflow and follow-up of oncology patients, including multimodality tumor tracking, characterization, and assessment. What's new is a fast, quantified 3D rendering protocol and analysis of tumor imaging for research into therapy response.
Other highlights in the company's ECR 2016 booth include its MobileDiagnost wDR, which will receive a sliding column in the near future, and MobileDiagnost M50, which will be released shortly and is being shown as a work-in-progress. In interventional x-ray, the company is demonstrating tailored interventional suites such as NeuroSuite, OncoSuite, and Hybrid Suite on its AlluraClarity angiography system. New innovations include EmboGuide and VesselNavigator, which improve 3D imaging to support interventional oncology.