Dissatisfied with their clinical PACS software, two radiology residents and a radiology attending physician at Stanford University decided to do something about it. Working nights and weekends, they developed ClariPACS, a Web-based PACS application designed for research and education purposes.
Based on HTML5, ClariPACS supports annotation and sharing of radiology studies and works with modern Web browsers, multiple computing platforms, and mobile devices such as the iPhone or iPad without installation of any software or plug-ins, said co-developer Dr. Ed Boas, a radiology resident at Stanford.
Interactive radiology reports can be produced with links to annotated key images and emailed, and full DICOM images can be embedded on a Web page. Users add images to ClariPACS by downloading the studies from their clinical PACS and then uploading the images to a ClariPACS server in the cloud.
"ClariPACS lets you share anonymized teaching cases outside your institution, which is not possible with a clinical PACS," Boas told AuntMinnie.com.
Other ClariPACS developers include Dr. Bao Do, a radiology attending at Stanford and the Palo Alto Veterans Affairs Hospital, and Dr. Mike Muelly, a radiology resident at Stanford.
Efforts to develop ClariPACS stemmed from the team's frustration over the clinical PACS software they've used, Boas said.
"Most of them look like they were designed in the 1990s, they don't work well on the Web, and there is no easy way for us to add new features," he said. "The clinical PACS manufacturers are really constrained in what they can do. Their systems have to be [U.S. Food and Drug Administration (FDA)] approved, and they have to work with legacy systems. We want to create better tools for radiologists, and the only way to do that was to create our own PACS."
Teaching and research
ClariPACS can be used, for example, in teaching and research applications such as online teaching modules and continuing medical education classes.
"Rather than just showing a handful of images, ClariPACS teaching modules let you scroll through the entire study, which provides more realistic training, especially for residents and less-experienced radiologists," Boas said.
The software could also be used for online journal articles. "Rather than static figures, the online versions of journal articles could include interactive figures with full DICOM datasets," he said.
ClariPACS could also enable multisite clinical trials to share imaging studies, according to Boas.
The software offers a number of key features, including automatic backups and security updates. Because the links to cases save the study layout, window/level settings, and annotations, email recipients can immediately see key findings, he said.
Cases can be password-protected or accessed publicly. Users can also search by diagnosis. Furthermore, ClariPACS performs HIPAA-compliant anonymization of teaching cases, Boas said.
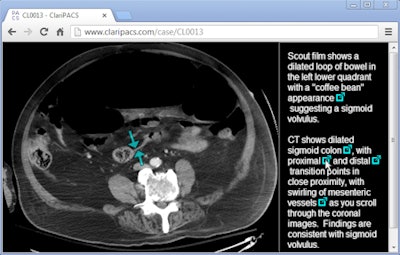 |
| Interactive radiology report in ClariPACS allows linking of annotated key images. The entire study and interactive report can be emailed to a colleague. Image courtesy of Dr. Ed Boas. |
Plug-ins allow new features to be added to ClariPACS; currently available plug-ins include support for thick slab reconstructions, maximum intensity projections (MIPs), and texture analysis.
Since ClariPACS formally launched in November 2012, the ClariPACS website has had 3,000 unique visitors, who have each viewed an average of seven cases, Boas said. In addition, 37% of visitors in December returned in January to view more cases.
"We currently have 90 public teaching cases and are adding more every week," he said. "Good content will be critical for getting more users."
Boas noted that Dr. Shawn Sato, a resident at the University of Iowa, recently developed a teaching module using ClariPACS. Boas said their software is also being evaluated by a large teleradiology services provider.
Clinical PACS?
While currently optimized as a teaching file and research PACS, ClariPACS may ultimately be able to serve as a clinical PACS, Boas said. ClariPACS has not received FDA clearance and does not interface with RIS, but the application's model of interactive radiology reports perhaps offers a preview of what clinical PACS could look like in the future.
"Today, radiology reports are reminiscent of the early days of the Web, when Web pages could only contain text," he said. "Images were added to Web pages in 1993 ... and now everyone takes it for granted that Web pages can contain images and interactive elements. ClariPACS shows how images and interactivity can be added to radiology reports."
Turning this vision into a clinical reality will require integration with existing RIS and HIS, as well as FDA clearance -- not a small task, Boas said. For now, the team is focused on creating better tools for radiologists, such as image postprocessing tools, computer-aided detection (CAD), and natural language processing tools.
"ClariPACS provides a platform for creating these tools," he said. "Initially, these will be used in a research environment, but eventually we will file for FDA clearance."