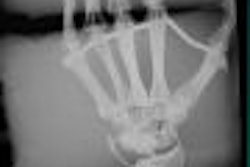
Biotechnology firm Luminetx of Memphis is using this week's American Healthcare Radiology Administrators (AHRA) show to demonstrate its VeinViewer product, which uses infrared technology to image veins.
VeinViewer employs a flexible head unit that can be positioned over any part of the anatomy and that beams infrared light onto the patient. The light makes veins visible to a digital video camera that transmits live video onto an image processor. The images are then sent through a projector that displays the image on the patient's skin, depicting veins within 0.6 mm of their actual location.
VeinViewer allows healthcare professionals to visualize the location and orientation of a patient's veins, enabling more accurate and precise entry for procedures such as phlebotomy, inserting IV or PICC lines, or sclerotherapy.
The technology was cleared by the Food and Drug Administration in 2004, and is undergoing clinical evaluation in physician offices in the U.S. and South America, according to the company.
Luminetx this week also announced a three-year distribution deal with Diomed Holdings, an Andover, MA-based developer of minimally invasive technologies. The deal gives Diomed exclusive rights in the sclerotherapy, phlebectomy, and varicose vein treatment markets in the U.S. and U.K. Diomed also agreed to make a $1 million investment in Luminetx.
By AuntMinnie.com staff writers
August 10, 2005
Copyright © 2005 AuntMinnie.com