Breast thermography vendor UE LifeSciences announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its NoTouch BreastScan device.
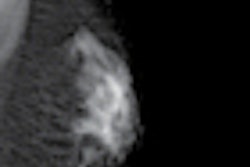
The product is a fully computerized functional infrared imaging system that may be used for adjunctive diagnostic screening for breast cancer.
NoTouch BreastScan dynamically analyzes temperature pixels from various infrared frames to create a real-time objective report for a physician. The system evaluates breast cancer from a physiologic vantage point not visible to imaging modalities such as mammography and ultrasound, according to UE LifeSciences.
The company plans to offer the NoTouch BreastScan device to gynecology, oncology, and radiology practices in the U.S., starting in the mid-Atlantic region. The product has already received CE Mark approval.