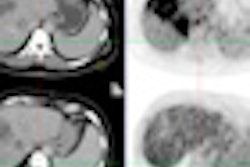
Crux Biomedical announced that it will present results from a recently completed trial of its Crux vena cava filter (VCF) system with bidirectional retrieval at the Society of Interventional Radiology (SIR) annual meeting in San Francisco.
The trial, titled Evaluation of the Crux Inferior Vena Cava Filter System (RETRIEVE), assessed the safety, performance, and efficacy of Crux's VCF system as both a retrievable and a permanent device. The trial included 125 patients at high risk for pulmonary embolism, and it was performed at 22 centers in the U.S., Australia, New Zealand, and Belgium, according to Crux Biomedical.
Crux VCF has received CE Mark approval for commercial use in Europe; it has been submitted to the U.S. Food and Drug Administration under an investigational device exemption study, the firm said.