The U.S. Food and Drug Administration (FDA) is recommending that patients who have implantable heart defibrillators that use St. Jude Medical's Riata and Riata ST leads receive x-ray or other imaging exams to check for abnormalities in the insulation surrounding the lead.
The abnormalities occur in the insulation around the electrical conductor wires, according to the FDA. St. Jude Medical stopped selling the leads in late 2010 and issued a recall in November 2011. According to the company, as of 2011, about 79,000 Riata leads remained implanted in patients living in the U.S.
Riata and Riata ST leads were used to connect an implantable cardioverter defibrillator or cardiac resynchronization therapy defibrillator to the heart to monitor its rhythms. When they detect life-threatening abnormal heart rhythms, they deliver an electrical shock to the heart to restore the rhythms to normal.
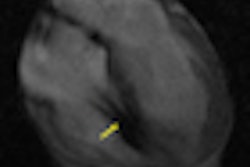
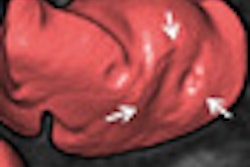
The leads for both defibrillators typically have layers of insulation surrounding them that protect electrical conductor wires inside the lead. Insulation failure may cause improper movement of some of the electrical conductors inside the leads; if this occurs, the lead may malfunction and result in inappropriate or no shock therapy, according to the FDA.
Routine imaging of the leads may detect previously unrecognized abnormalities in the insulation. X-rays or other imaging techniques will help healthcare providers develop individualized patient treatment plans, the FDA said.
The FDA cautioned against routine removal of leads without careful evaluation of benefits and risks to the individual patient. The agency is also requiring St. Jude Medical to conduct three-year postmarket surveillance studies to collect clinical data related to premature insulation failure in Riata and Riata ST leads.