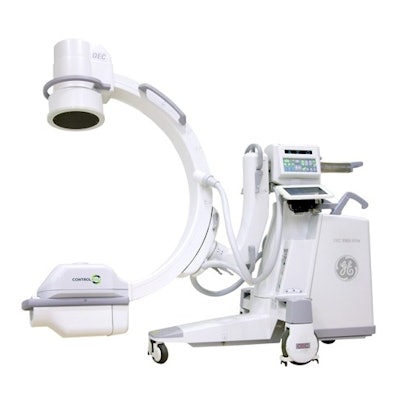
ControlRad has received 510(k) clearance from the U.S. Food and Drug Administration for the marketing of its Trace fluoroscopy radiation dose reduction technology with GE OEC 9900 Mobile C-arms from GE Healthcare.
Trace can be integrated on existing mobile C-arms and utilizes a proprietary semitransparent filter, tablet along with enhanced image-processing techniques to lower radiation dose for any fluoroscopic imaging procedure. ControlRad received the original clearance for Trace in 2019.
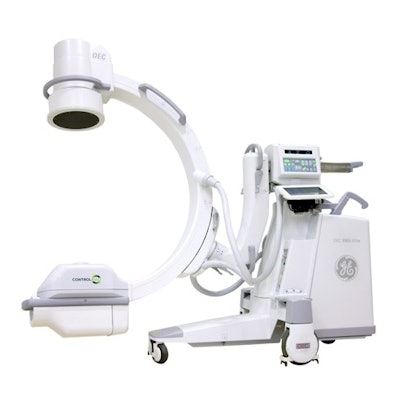 ControlRad Trace Model 9 installed on the GE OEC 9900 Elite mobile C-arm. Image courtesy of ControlRad.
ControlRad Trace Model 9 installed on the GE OEC 9900 Elite mobile C-arm. Image courtesy of ControlRad.The clearance will allow ControlRad to sell Trace as an add-on to GE OEC 9900 Mobile C-arms installed in the field.
In other news, ControlRad announced that it had completed a series C financing led by existing investor Questa Capital. The financing includes new investor Windham Ventures.