The U.S. Food and Drug Administration (FDA) has cleared x-ray developer Nanox's single-source Nanox.ARC digital x-ray. Nanox plans to start shipping the system in the fourth quarter of 2021.
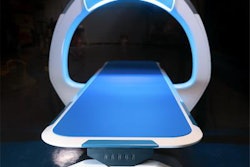
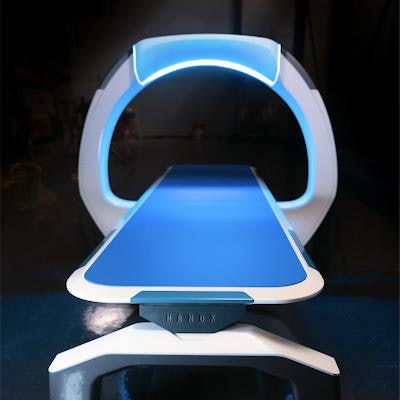
 The Nanox.ARC digital x-ray system. Image courtesy of Nanox.
The Nanox.ARC digital x-ray system. Image courtesy of Nanox.The 510(k) clearance comes as a result of FDA asking for more information in February to address "certain deficiencies and questions, including requests that the company provide additional support regarding the intended use of the Nanox.ARC and the comparability of the Nanox.ARC to the predicate device."
The clearance means Nanox is on target to start shipping in the fourth quarter of this year, with the goal of deploying 15,000 Nanox.ARC systems by the end of 2024, Nanox said.
In other Nanox news, the firm plans to submit a 510(k) application to the FDA for its multisource Nanox.ARC system and Nanox.CLOUD service later this year.