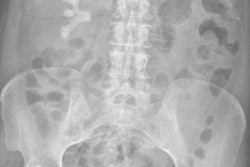
The National Council on Radiation Protection and Measurements (NCRP) on January 13 advised against the routine use of gonadal shielding during abdominal and pelvic radiography. The change is in line with current scientific thinking on the risks of shielding, such as missed findings.
The NCRP explained the new recommendation and the background on gonadal shielding in a statement, and it provided guidance for carrying out changes in an implementation document.
"NCRP now recommends that [gonadal shielding] not be used routinely during abdominal and pelvic radiography, and that federal, state, and local regulations and guidance should be revised to remove any actual or implied requirement for routine [gonadal shielding]," the agency said in the statement. "[Gonadal shielding] use may remain appropriate in some limited circumstances."
It also provided a trifold flyer with information for patients, which noted that the new policy has the support of major organizations such as the American College of Radiology, the American Society for Radiologic Technologists, and the American Association for Physicists in Medicine (AAPM).
Gonadal shielding has been widely used since the 1950s as a means of protecting patients from radiation damage to the testes and ovaries that could be passed on to future generations. However, scientific knowledge on the subject has evolved.
"The risks of heritable genetic effects are now considered to be much less than previously estimated," the NCRP noted. "Improvements in technology since the 1950s have resulted in up to a 95% reduction in the absorbed dose to pelvic organs from radiography."
Furthermore, automatic exposure control on today's equipment may adjust to shielding by increasing radiation dose, creating risk for other organs, some of which are more sensitive than the gonadal areas. Shielding may also make it difficult to visualize pelvic organs and, if results are inconclusive, repeat exams may be needed.
In any case shielding may not actually protect gonadal areas because the location of gonads "varies considerably" among patients and patient movement may also minimize its value.
"As a result, NCRP has concluded that in most circumstances [gonadal shielding] use does not contribute significantly to reducing risks from exposure and may have the unintended consequences of increased exposure and loss of valuable diagnostic information, and therefore use of [gonadal shielding] is not justified as a routine part of radiological protection," the agency noted.
State regulations have been in line with regulations of the U.S. Food and Drug Administration (FDA) from 1976, which require gonadal screening during abdominal and pelvic radiography, with exceptions in some clinical cases. However, last April, the FDA proposed removing requirements for shielding.