SINGAPORE - A deep-learning AI model that can “translate” cine MRI into images resembling those in late gadolinium enhancement (LGE) could reduce the use of contrast agents in patients, according to a presentation at the International Society of Magnetic Resonance in Medicine (ISMRM) 2024 annual meeting
Pengfang Qian, PhD, of ShanghaiTech University in China noted that their new algorithm technique could have applications in patients with acute myocardial infarction.
“This study presents an effective, non-invasive, and contrast-free method for predicting LGE in acute [myocardial infarction], potentially reducing the use of gadolinium-based contrast agents and shortening cardiac MR examinations,” Qian noted.
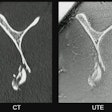
In cardiac MRI, late gadolinium enhancement (LGE) is a standard tool widely used for diagnosing various myocardial diseases, primarily by detecting injured areas such as scars in the heart muscle. LGE can detect acute myocardial infarction, for instance, yet the technique can’t be used in patients with contraindications to gadolinium contrast agent, Qian noted.
Hence, the researchers explored the feasibility of generating LGE images using only contrast-free cine images (images captured at a rapid rate over time) and in doing so developed a novel technique they’ve called “Cine Generated Enhancement” (CGE).
In the presentation, Qian discussed developing and validating the approach in a group of acute myocardial infarction patients, with the study comparing scar size and extent of the injury (transmurality) in patients on CGE images and standard LGE images.
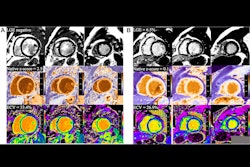
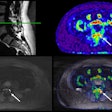
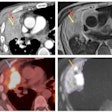
 Visualization CGE of varying generated contrasts (gray, intermediate and bright blood) compared with LGE for two AMI patients. (A) A patient with large transmural LGE; (B) A patient with tiny LGE on basal slices.Image courtesy of Pengfang Qian, PhD
Visualization CGE of varying generated contrasts (gray, intermediate and bright blood) compared with LGE for two AMI patients. (A) A patient with large transmural LGE; (B) A patient with tiny LGE on basal slices.Image courtesy of Pengfang Qian, PhD
In brief, a crucial step involved first aligning the contrast-free cine and LGE images using automatic slice-by-slice pairing and registration between them based on imaging geometry parameters. These pairs were then used to train a generative adversarial neural network to “translate” cine images into images resembling LGE.
Next, the researchers compared the quality of these CGE images to standard LGE imaging in a hold-out data set from 30 patients with myocardial infarction. Qian presented an image (below) of the results in two patients that he said highlights good agreement of enhanced myocardium between CGE and LGE across the left ventricle for various CGE contrasts. Overall, CGE closely resembles LGE for the two patients, while part of the scar in the apical slices of one patient (Fig. 2A) was only moderately enhanced, he noted.
According to the group’s statistical analysis, there was a significant (p < 0.0001) correlation between CGE and LGE for measuring scar size and transmurality. A Bland-Altman analysis indicated however that the CGE images slightly underestimated these markers, Qian noted.
“Realistic enhancement images can be generated for acute [myocardial infarction] patients using cine images unseen during training," Qian said. "The scar size and transmurality estimated with CGE agreed well with LGE."
Future work will explore postprocessing corrections to address the scar underestimation of CGE and expand the patient population for further validation, Qian concluded.