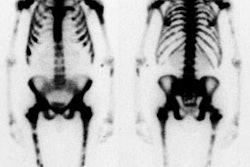
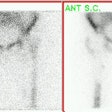
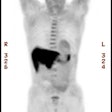
Prognostic Value of Thallium Exam
In general, patients with 3-vessel disease and left ventricular rest dysfunction, multivessel disease and exercise induced left ventricular dysfunction, or multivessel disease associated with left main disease have the worst prognosis. Surgical intervention in these patients can translate to a marked improvement in survival. Coronary angiography does not add significant prognostic information to the thallium exam.
Bayesian Anaysis
(Pre-Test Likelihood of Disease)
The likelihood of coronary artery disease can range from as high as 93% in men with definite angina, to as low as 6% in women with non-specific chest pain. There is little benefit (ie: additional information regarding the presence of disease) from thallium scintigraphy when the actual disease prevalence is very low or very high. In other words, in someone with a very high pretest probability of disease, a negative test is more likely to represent a false negative. The greatest benefit of a negative or positive exam is when there is a moderate likelihood of disease (25-75%).
Coronary Artery Disease
For both exercise and pharmacologic stress, an increased risk for subsequent cardiac events is indicated by the the presence of reversible thallium defects, the number of reversible defects, large defects, an abnormal heart to lung ratio, or transient stress dilatation of the left ventricle. In general, patients with reversible perfusion defects have a higher incidence of cardiac events during follow-up than do patients with fixed defects. Patients with moderate to severe ischemia involving more than 10% of the myocardium have been shown to have an increased survival benefit from revascularization compared to medical therapy alone [17].
It is important to note that even in the face of angiographically detected CAD, a normal thallium stress exam is associated with a yearly rate for cardiac events of less than 1% [1,2,3]. The cardiac event rate is slightly higher despite normal images in patients with known coronary artery disease or prior revascularization. In a large study of patient's using primarily thallium images (75% of exams) the annual mortality rate for patient's with normal perfusion scans was 1.9% [20]. Importantly, only 1.3% of patients with normal scans were referred for coronary angiography (based upon clinical or exercise findings worrisome for CAD)- indicating that SPECT imaging is an effective gatekeeper for angiography [20]. Of patients with normal exams that underwent angiography, one-third were found to have had CAD- hence, SPECT imaging results are not infallible and all factors must be considered when evaluating patients with suspected CAD [20].
It is well appreciated that the diagnostic accuracy of myocardial perfusion imaging in women can be adversely affected by gender-specific factors such as breast attenuation, a small left ventricular chamber size, and a high prevalence of single vessel disease [13]. None-the-less, pooled data would suggest that women with a normal myocardial perfusion exam have an annual cardiac event rate of less than 1% and that an abnormal exam is associated with an increased risk for cardiac events [13].
Post Myocardial Infarction Imaging
Factors which have been shown to affect infarct size include:
- The amount of myocardium at risk (most important determinant)
- Collateral flow to the infarct zone
- Time to reperfusion.
The extent of myocardial salvage is best if reperfusion is accomplished within one hour. Between 4 to 6 hours the chance of significant myocardial salvage diminishes greatly. After 12 to 24 hours one can assume that the infarction is complete and that no salvageable myocardium is present. In general, patients with anterior ST-segment elevation on ECG have twice the myocardium at risk (approximately 50% of the left ventricle) as do patients with inferior or lateral ST elevation (about 20% of the left ventricle).
As a corollary, inferior infarctions seem to have a smaller percentage of myocardium salvaged following thrombolytic therapy in comparison to anterior wall infarctions. Patients without ST-segment elevation have a high prevalence of left circumflex occlusion which is often not identified on the ECG. Nonetheless, the actual amount of myocardium at risk is highly variable from patient to patient. In non-transmural infarction, the regional uptake of thallium is reduced in proportion to the volume of myocardial necrosis, and the defect intensity remains unchanged on the redistribution image. Despite the close correlation between thallium uptake after reperfusion and subsequent improvement in regional wall motion, early post-reperfusion thallium uptake appears to overestimate myocardial viability. This may occur because in the period soon after reperfusion, the distribution of thallium may reflect hyperemic flow, thereby overestimating the extent of myocardial salvage [6]. Thus, early thallium uptake after reperfusion cannot reliably differentiate viable from necrotic myocardium. However, necrotic myocardium cannot retain thallium and despite its initial uptake, thallium washout is accelerated from these areas- this produces a pattern of "Reverse Redistribution".
Sub-Acute Phase/At Hospital Discharge;
Perfusion imaging performed soon after an acute MI can effectively predict the risk of future cardiac events [19].
During the subacute phase, most patients have an uncomplicated course, however, some patients (up to 20%) may experience recurrent ischemia or re-infarction. Adenosine or dipyridamole imaging during the 2nd to 5th day post-MI can provide early risk stratification. In hospital cardiac events occurred in 45% of patients who demonstrated evidence of residual ischemia in the infarct zone. No events were observed in patients without evidence of ischemia. Thus, imaging can be used to identify a subset of patients who may benefit from more aggressive management (ie: those with evidence of ischemia).
At discharge about 35% of post-MI patients with an uncomplicated hospital course will demonstrate scintigraphic evidence of ischemia. Three parameters of myocardial perfusion imaging which are strong predictors of future cardiac events in post-MI patients are: 1- The presence of transient defects; 2- The number of transient defects; and 3- Increased lung uptake. Patients with a fixed, single thallium defect at the time of discharge, have about a 6% incidence of cardiac events on one year follow-up. A reversible perfusion defect identified in the acute post-MI setting is a strong predictor of future cardiac events. Prognosis is related to both myocardial infarct and ischemic defect extent (size) and severity. The larger the area of ischemia, the more severe the defect, or the greater the number of reversible defects, the worse the prognosis (up to a 51% incidence of cardiac events on one year follow-up). An abnormal heart to lung ratio on post exercise imaging, however, is probably the most significant poor prognostic indicator.
On post MI adenosine thallium imaging, patients with large fixed perfusion defects (over 20% of the LV) and large areas of ischemia (over 10% of the LV) have a high cardiac event rate and that rate increases in proportion to the perfusion defect size [19]. These patients require aggressive treatment with intensive medical therapy and invasive evaluation in order to reduce their risk of subsequent cardiac events [19].
LVEF is also an important indicator of long term survival in post-MI patients. Prognosis changes very little within the range of LVEF's from 45 to 65%, however, once resting LVEF drops below 40%, mortality increases exponentially [7,8].
Unstable Angina
In unstable angina, perfusion defects can persist for hours after resolution of chest pain symptoms. Rest thallium imaging in patients with unstable angina has demonstrated reversible perfusion defects in 40 to 75% of patients. Defects are observed less frequently if the tracer is injected more than 12 hours after resolution of the patients symptoms. The presence of such defects are associated with an increased risk for subsequent cardiac events. Dipyridamole thallium imaging can also provide diagnostic and prognostic information in this group of patients [6].
Tc-Sestamibi imaging may also be useful in these patients as an injection of the tracer can be made when the patient is experiencing chest pain, but imaging defered until the patient is stabilized. A normal exam is associated with a 0 to 4% risk of CAD. Additionally, in patients with a normal scan, the risk for subsequent cardiac event in the next year varies between 0 to 2% [6].
Evaluating Efficacy of Revascularization
Following PTCA
A scan after PTCA is often obtained to assess for improvement in perfusion to the ischemic zone, to determine the risk of restenosis, and to serve as a comparison study in order to detect silent restenosis. (Up to 20-45% of successful PTCA procedures are complicated by restenosis within 6 months). Scintigraphy is superior to exercise ECG for detecting restenosis [6].
Post PTCA scans should be delayed for at least 2 to 4 weeks after the procedure. Scans performed before this time can reveal apparent fixed defects or are falsely positive for ischemia- these findings may be related to transient persistent myocyte dysfunction or spasm/endothelial damage at the site of the procedure.
PTCA patients who demonstrate evidence of ischemia on exercise perfusion imaging performed 2 to 4 weeks following the procedure are at a markedly increased risk for developing restenosis (40-75%), whereas only about 15% of patients with a normal study will develop restenosis. Although the accuracy of the test is superior to the exercise ECG, routine use of scintigraphy as a screening procedure has not been shown to be cost effective [6].
Following CABG
After CABG, occlusion of venous grafts occurs in up to 10% before hospital discharge, up to 20% at 1 year, and up to 50% at 10 years. Normalization of the patients scan post-operatively is associated with an 80% graft patency rate, while the presence of a new or worsening perfusion defect is associated with a patency rate of between 15 to 54% [6].
Preoperative Evaluation:
The American Heart Association and the American College of Cardiology have guidelines for the preoperative clinical assessment of patients at risk for perioperative cardiovascular complications [18]. Additionally, patients who cannot perform at least 4 METS of work (light work around the house, walking on level ground at 4 mph, or climbing a flight of stairs without stopping) are also at increased risk for perioperative cardiovascular events [18].
| Major Risk Factors | Intermediate Risk Factors | Minor Risk Factors |
| Unstable coronary syndromes: Acute or recent MI | Mild angina (Canadian class I or II) | Advanced age |
| Decompensated heart failure | Previous MI by history or Q-waves | Abnormal ECG: LV hypertrophy, LBBB, ST-T abnormalities |
| Significant arrhythmia: High garde AV block, supraventricular arrhythmia with uncontrolled ventricular rate, symptomatic ventricular arrhythmia in the presence of underlying heart disease | Compensated or prior heart failure | Rhythm other than sinus: atrial fibrillation |
| Severe valvular heart disease | Diabetes (particularly insulin dependent) | Low functional capacity or history of stroke |
| Renal insufficiency | Uncontrolled systemic hypertension |
For patients with major clinical risk predictors, the guidelines recommend delaying surgery, optimizing risk factor modification, and possibly performing coronary angiography [18]. In patients with intermediate and minor predictors, the guidelines recommend non-invasive testing [18].
Vascular Surgery Preoperative Evaluation
Perioperative cardiac events carry an poor outcome with mortality between 26% to 70% [16]. There is a high incidence of coronary artery disease in patients scheduled for elective vascular surgery. Up to 35% of asymptomatic patients pre-op for peripheral vascular disease will have at least 1 vessel coronary artery disease. Two factors which are associated with an increased risk for perioperative cardiac events include the presence of stress-induced ischemia and the patients left ventricular ejection fraction. Both thallium and technetium agents have been used for preoperative cardiac evaluation. The ACC and AHA recommend preoperative cardiac exercise or pharmacologic stress imaging for patients with an intermediate pretest probability of CAD [21].
Stress induced ischemia and perioperative risk:
In general, the risk of perioperative cardiac events is directly related to the extent of reversible perfusion defects identified. Revascularization of these patients can reduce their peri-operative risk for cardiac events [16]. Assessment for the presence of certain clinical variables can further help to detect those individuals at greatest risk for perioperative myocardial complications. These variables can also identify those patients in which myocardial perfusion imaging will provide the most useful clinical information.
Some clinical variables which should be evaluated in each pre-op vascular surgery patient are: ECG evidence of a Q-wave (history of myocardial infarction); age over 70; classic angina; treated diabetes mellitus; treated PVC's or cardiac arrhythmia; congestive heart failure; prior coronary bypass surgery; and hypertension [12,21]. The Lee criteria developed in 1999, include high risk type of surgery; history of ischemic heart disease; history of CHF; history of cerebral vascular disease; preoperative treatment with insulin; and preoperative creatinine greater than 2.0 mg/dL [21].
Patients with no variables have a very low incidence of post-op cardiac complications so imaging is unlikely to provide additional clinical information given the patient's low risk for perioperative cardiac events. Patients with 3 or more clinical variables can have up to an 11-18% incidence of post-op cardiac complications and should undergo additional imaging [21]. Patients with intermediate risk that undergo major vascular surgery can be kept at a low perioperative risk by optimization of medical treatment with beta-blockers and tight heart rate control [21].
In the sub group of patients with one or two clinical risk factors, if ischemia was detected on the stress thallium scan, there was a 30% incidence of cardiac complications; whereas there was only a 6% incidence of perioperative complications in patients with a normal thallium exam [9]. The one drawback of this study was that one-third of patients underwent non-aortic surgery (endarterectomy or fem-pop bypass) which tends to carry a lower overall perioperative cardiac risk. Thus, for patients undergoing non-aortic vascular surgery, the above listed clinical criteria may be very useful in selecting patients who require further risk stratification. Additional caution, however, may be warranted in patients who are to undergo higher risk aortic surgery [10].
Unfortunately, there is no consensus regarding the utility of pre-operative stress cardiac imaging and some authors [11] have concluded that pre-operative perfusion scan evaluation did not provide useful information regarding the risk of peri-operative cardiac events in patients scheduled for abdominal aortic surgery. Age over 65 and definite clinical evidence of coronary artery disease were the best predictors of cardiac complications (40% incidence in patients with both risk factors, 24% with one risk factor), while age over 65 years was the best predictor of post-operative mortality. Radionuclide determined LVEF less than 50% was predictive of perioperative left ventricular failure.
The bottomline is that patients with an intermediate clinical risk for perioperative events, those with a low functional capacity, patients undergoing high-risk non-cardiac surgery, or those patients with a high surgical risk may benefit from preoperative perfusion imaging [16]. The presence of reversible defects is associated with a higher risk for peri-operative cardiac events and the greater the number of reversible defects, the greater the risk [12]. A normal preoperative stress myocardial perfusion imaging exam (using either thallium or a technetium agent) provides excellent negative predictive value (99%) and is associated with a very low likelihood for peri-operative cardiac events [12,14,16].
LVEF, wall motion analysis and perioperative risk:
A LVEF of less than 50% on gated perfusion imaging is associated with an increased risk for perioperative cardiac events. The event rate increases with decreasing EF value [12]. Patients with wall motion abnormalities on QGS evaluation are also at an increased risk for perioperative cardiac events [12].
REFERENCES:
(1) New Engl J Med 1993; Zaret BL, Wackers FJ. Nuclear cardiology (1). 329: 775-783
(2) Am J Cardiol; Cont. Ed. Series. Part III. p.3-10
(3) J Nucl Med 1994; Maddahi J. Cardiovascular nuclear medicine: state-of-the-art. 35: 672-73
(4) Chest 1999; Mandalapu BP, et al. Technetium Tc 99m Sestamibi myocardial perfusion imaging. Current role for evaluation of prognosis. 115: 1684-1694
(5) J Nucl Med 1994; Brown KA. The role of stress redistribution thallium-201 myocardial perfusion imaging in evaluating coronary artery disease and perioperative risk. 35: 703-06
(6) Semin Nucl Med 1995; Newhouse HK, Wexler JP. Myocardial perfusion imaging for evaluating interventions in coronary artery disease. 25: 15-27
(7) J Nucl Med 1994; Verani MS. Exercise and pharmacologic stress testing for prognosis after acute myocardial infarction. 35: 716-20
(8) J Nucl Med 1994; Port SC. The role of radionuclide ventriculography in the
assessment of prognosis in patients with CAD.
35: 721-25
(9) Ann Intern Med 1989; Eagle KA, et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery.110: 859-866
(10) Am J Cardiol; Cont.Ed.Series. Part III. p.16-21
(11) New Engl J Med 1994; Baron JF, et al. Dipyridamole-thallium scintigraphy and gated radionuclide angiography to assess cardiac risk before abdominal aortic surgery. 330: 663-69
(12) J Nucl Med 2003; Hashimoto J, et al. Preoperative risk stratification using stress myocardial perfusion scintigraphy with electrocardiographic gating. 44: 385-390
(13) J Nucl Cardiol 2003; Mieres JH, et al. A report of the American society of nuclear cardiology task force on women and heart disease (writing group on perfusion imaging in women). 10: 95-101
(14) J Nucl Cardiol 2003; Cohen MC, et al. Perioperative and long-term prognostic value of dipyridamole Tc-99m sestamibi myocardial tomography in patients evaluated for elective vascular surgery. 10: 464-472
(15) J Nucl Cardiol 2003; Elhendy A, et al. Risk stratification of patients after myocardial revascularization by stress Tc-99m tetrofosmin myocardial perfusion tomography. 10: 615-622
(16) J Nucl Cardiol 2004; Brown KA. Advances in nuclear cardiology: preoperative risk stratification. 11: 335-348
(17) J Nucl Cardiol 2005; Shaw LJ, et al. Computed tomography imaging within nuclear cardiology. 12: 131-142
(18) J Nucl Cardiol 2005; Pryma DA, et al. Cardiovascular risk assessment in cancer patients undergoing major surgery. 12: 151-157
(19) J Nucl Cardiol 2005; Dakik HA, et al. Prognostic value of adenosine Tl-201 myocardial perfusion imaging after acute myocardial infarction: results of a prospective clinical trial. 12: 276-283
(20) J Nucl Cardiol 2007; Miller TD, et al. A normal stress SPECT scan is an effective gatekeeper for coronary angiography. 14: 187-193
(21) J Nucl Cardiol 2007; Hoeks SE, et al. Preoperative cardiac testing before major vascular surgery. 14: 885-891