Bone Imaging in Neoplastic
Disease
Malignant
Bone Tumors:
Metastatic
Bone Malignancies:
According to the Radiologic Clinics of North America
(July 93), bone scan is 50 to 80% more sensitive than
radiographs in detecting skeletal metastases. This is probably
because about 30-75% of the bone mineral content must be lost
before a metastasis is evident on a radiograph [11]. In
contrast, as little as a 5-10% change in the ratio of the lesion
to normal bone is required to detect an abnormality on bone scan
[11]. The sensitivity for bone scan in detecting bone metastases
is between 62% - 100% [11]. For myeloma or very osteolytic aggressive lesions, however, bone
scan is less sensitive- as low as 50% for multiple myeloma [24],
as compared to an 80% detection on
skeletal survey. Because of this, skeletal scintigraphy
is probably not the procedure of choice for evaluating the
presence of skeletal involvement in patients with multiple
myeloma or lymphoma.However, SPECT/CT can be used to improve the
detection of lytic bone lesions [24].
The specificity of the bone scan in the evaluation of metastatic disease is unfortunately limited as fractures, degenerative disease, or other benign conditions may produce false-positive examinations. Nonetheless, given the ease of performing a whole body survey, bone scan is probably the study of choice for the initial evaluation for mets in patients with cancer.
SPECT imaging results in overall improved sensitivity with the detection of 20-50% more lesions in the spine when compared to planar images [11]. SPECT-CT can further improve accuracy by improving anatomic localization and by permitting better evaluation for underlying bone pathology [13].
About 90% of patients with skeletal metastases present with multiple lesions. Nearly 80% of all metastatic lesions are in the axial skeleton. In patients with a known malignancy, 60 to 70% of axial lesions are due to metastases, whereas about 40 to 50% of lesions in the extremities or skull are due to mets. A solitary rib lesion has about a 10% probability of representing a met in a patient with a known malignancy [1]. In the evaluation of vertebral lesions, there are certain characteristics of the tracer distribution which can aid in scan interpretation- particularly when SPECT images are performed. Abnormalities that extend beyond the vertebral body are invariably due to osteophytes [10]. Uptake confined only to the articular facets is nearly always benign [10]. Tracer uptake involving both the vertebral body and pedicles is usually indicative of metastatic disease [10].
Other authors have concluded that a single lesion has about a 11% probability for being a met in
patients with known underlying malignancy (in this study the
underlying malignancy was breast cancer). The percentage
increased to 35% when 2 new lesions were detected, and reached
100% when 5 new lesions were identified [2]. In multifocal
metastatic disease the regional distribution of lesions for
common bone seeking primaries (such as breast, lung, and
prostate cancer) is: thorax/ribs (37%), spine (26%), limbs
(15%), pelvis (15%), and skull (6%).
Following successful treatment, bone metastases improve slowly
on imaging [24]. A "flare" phenomenon (described below) can
result in the appearance of disease progression [24].
Additionally, certain chemotherapeutic agents can directly
interfer with bone remodeling [24]. For example, the anti-RANKL
(receptor activator of nuclear factor kB ligand) monoclonasl
antibody denosumab inhibits osteoclast function which interferes
with the ability to accurately assess response on bone scan
[24]. In patients on this agent, the bone scan can appear to
normalize with resolution of previously noted areas of tracer
uptake suggesting a positive therapeutic response, despite the
presence of residual metastatic disease [24].
The 'Flare phenomenon' reflects a favorable response of bone metastases to treatment. Patients are typically asymptomatic and plain films generally show sclerosis of the lesions [10]. The phenomenon is typically seen between 2 weeks to 3 months following therapy, but can rarely be seen as late as 6 months after treatment. Continued increase in the number and intensity of lesions beyond 6 months is usually indicative of disease progression [10]. A flare response can also be seen with 18F-fluoride PET bone scanning [12]. In general, it is prudent to wait about 3 months following completion of a new therapeutic intervention prior to repeating the bone scan. The diagnosis of "flare" requires 2 criteria:
- Increased
intensity and/or number of lesions on bone scan (Felt to be
secondary to increased osteoblastic
activity associated with healing)
- Subsequent decrease uptake in these lesions on repeat exam in 2-3 months.
Bone
Scan in Breast Cancer:
The skeleton is the most common site of distant metastases in breast cancer [11]. Bone scintigraphy is more sensitive in detecting metastatic breast carcinoma than plain films, with an average of between 4 to 6 months for conversion of the radiograph after identification of the lesion by bone scan. In breast cancer patients, there is a low positive yield for the bone scan in patients with early stage disease: About 4% (0-18%) for Stage I and 7% (0-32%) for Stage II disease. In patients with Stage III disease, the yield may be as high as 14 to 28%. In stage IV disease, bone metastases can be found in up to 40.5% of patients [11]. In patients who have small primary tumors (less than 2 cm), the routine use of preoperative bone scanning in asymptomatic patients is probably unnecessary [3]. Coleman et al (JNM 1988), recommend a baseline bone scan for all patients with Stage II-IV disease. Women with breast cancer who present with bone mets at the time of diagnosis, have a markedly worse prognosis. In patients with breast cancer, 21% of patients relapsed with a solitary bone met [35], the most common location being the spine. Although bone pain is a common presenting symptom in these patients, up to 30 to 50% of these patients can be asymptomatic. It is important to note that an isolated sternal lesion in a patient with breast cancer has a 75% likelihood of being a met. There is also a strong correlation between the side of the sternal met, the side of the primary breast lesion, and the presence of pathologic internal mammary lymph nodes.
Following initiation of therapy, up to 75% of patients with breast cancer show increased activity or new lesions due to bone repair at responding sites of metastatic disease [12].
Bone
Scan in Prostate Cancer:
In a meta-analysis, bone scan/SPECT had a per patient combined
sensitivity of 79% and a specificity of 82% for the detection of
metastatic disease [39]. The per lesion sensitivity and
specificity were 59% and 75%, respetively for planar imaging,
and 90% and 85%, respectively for SPECT imaging [39]. The
likelihood for a positive scan is also dependent on the
patient's PSA level [39]. In a separate meta-analysis, the
incidence of bone metastases by bone scan in patients with newly
diagnosed prostate cancer was 2.3% in patients with PSA less
than or equal to 10 ng/mL, 5.3% in patients with PSA between
10.1-19.9 ng/mL,
and 16.2% in patients with PSA levels between 20-49.9 [11]. The
likelihood for bone metastatses
increases to about 50% when the PSA level is greater than 50 ng/mL [15]. However, on 68Ga-PSMA
imaging, at least one study found bone metastases at initial
staging in 17% of patients with PSA levels below 5 ng/mL [40].
Based upon the Gleason score, detection rates are 5.6% of
patients with a score of less than or equal to 7, and 29.9% of
patients with a score of 8 or greater [11].
The prevailing consensus is that in newly diagnosed
asymptomatic patients with prostate cancer and a low PSA (less
than 20) there is a very low likelihood (less than 1%) of having
bone metastases [11]. Other authors suggest that a PSA level of
less than 10 ng/mL in a patient with a well or moderately
differentiated tumor (and the absence of specific bone symptoms)
has a 99% negative predictive value for bone metastases [35].
Guidelines vary, but in general, bone scan should be limited to
patients for initial staging with a T-stage greater than 2,
elevated PSA (greater than 10 or 20 ng/mL), high Gleason scores (8-10), locally
advanced disease, elevated alkaline phosphatase,
or bone symptoms [9,11,15,37]. Other
authors suggest screening with bone scintigraphy for patients
with PSA levels over 20 ng/mL [35]. Patients who are receiving
hormonal therapy (antiandrogen) may
have bone metastases, even when their PSA is normal. In patients
that are treated with tyrosine kinase inhibitors (such as
sunitinib) for castration resistant prostate cancer, the bone
scan findings may dramatically improve, but other indicators of
disease such as PSA and other sites of disease on CT, may not
improve [23].
The Prostate Cancer Working Group criteria defines disease
progression on bone scan as requiring at least 2 new lesions of
the first assessment after a baseline bone scan and then at
least 2 further lesions on a subsequent confirmatory bone scan
[41].
|
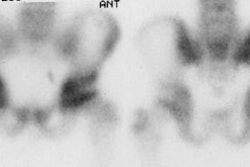
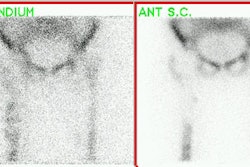
"Superscan" in patient with prostate cancer: A "Superscan" occurs in a patient with widespread osteoblastic bone metastatses. There is diffuse, intense skeletal uptake of the tracer with absent renal and background activity. |
|
|
Bone Scan in Lung Cancer: Hypertrophic
Osteoarthropathy:
Hypertrophic osteoarthropathy can be primary or secondary [34].
Primary HOA is a rare hereditary disease with either autosomoal
dominant or autosomal recessive inheritance [38]. There are
three subtypes of primary HOA- classic, incomplete, and forme
fruste [38]. The classic form presents with both pachydermia and
periostitis [38]. The incomplete subtype is the most common and
presents with isolated periostitis [38]. The forme fruste
presents with isolated pachydermia- onset occurs around puberty
and is more common in males and African Americans [38].
Secondary HOA is the most common form, and accounts for 95-97%
of cases [34]. Secondary HOA is most commonly associated with
intrathoracic disease [34] (lung cancer, bronchiectasis, cystic
fibrosis, mesothelioma, fibrous tumor of the pleura,
interstitial lung disease, pneumoconiosis), but can also occur
in association with cyanotic heart disease, and inflammatory
bowel disease.
The most common cause of HOA is non-small cell lung cancer
[34]. HOA occurs in about 10% of
patients (4% to 17% [34]) with bronchogenic
carcinoma and consists of 2 components: 1- Clubbing of the
fingers and toes; and 2- Periostitis
of the long bones. Patients may have pain and swelling about the
wrists, and less commonly the ankles
and knees.
Plain film findings in HOA reveal a smooth, thick, linear,
lamellar periosteal new bone
formation along the shafts of tubular bones sparing the
epiphyses (epiphyseal involvement is more common with primary
HOA [34]). The tibia, fibula, radius, and ulna are the most
commonly affcted bones, followed by the phalanges of the fingers
[34]. Bone scan may show symmetric patchy or linear increased
activity along the periosteum of
long bones- termed the tram line or double stripe sign [34].
Both 18F-fluoride and FDG uptake has been reported
corresponding to the periosteal thickening [34]. HOA tends to
resolve following tumor resection.
Differential considerations for HOA include:
- Thyroid acropachy: The condition arises after treatment for Graves disease, including ablation or resection [34]. Patients will also present with digital clubbing, pretibial myxedema, and exophthalmos [34]. The autoimmine process underlying Graves disease also stimulates fibroblasts in bones [38]. The periosteal reaction of thyroid acropachy is typically lacy, fluffy, spiculated, and thick, involving the diaphyses of short tubular bones in the hands and feet, commonly involving the radial side of the 1st, 2nd, and 3th metacarpals/metatarsals, the ulna aspect of the 4th and 5th metacarpals/metatarsals, and proximal and middle phalanges of the fingers [34,38]. Unlike with HOA, the tibia, fibula, radius, and ulna are not usually involved [34].
- Voriconazole-induced periostitis: The agent is a second generation triazole antifungal commonly used to treat immunocompromised (organ transplant) patients for invasive aspergillosis and candidemia [34]. The patients present with refractory joint pain [34]. The induced periostitis is usually patchier and more asymmetric compared to HOA, and can involve the clavicles, ribs, scapula, acetabulum, and hands [34]. The periosteal reaction appears dense, focal, nodular, and irregular, as opposed to the smooth or linear periostitis in HOA [34].
- Venous insufficiency: Long-standing impaired venous return may elicit a periosteal reaction in the tibia and fibula due to increased pressure on the periosteum [34]. The reaction can initially be separate from the cortex and usually appears thick, undulating or shaggy, and irregular, although like HOA, it is usually symmetric [34]. Bone scan will also show retained lower extremity soft tissue activity due to impaired clearance [34].
- Hypervitaminosis A: The condition results from overuse of retinoids in adolescent and preadolescent children with acne, psoriasis, or burn injuries [34]. Besides periostitis, other symptoms of hypervitaminosis A include nausea, vomiting, osteoporosis, arthralgia, myalgia, and muscle weakness [38]. There is a thick, dense, wavy periosteal reaction usually greatest near the diaphysis and tapering towards the ends of the bone [34]. The most commonly involved bones are the ulna and metacarpals/metatarsals, followed by the clavicle, tibia and fibula [34,38]. Cortical thickening of the tubular bones may also occur [34].
The periostitis is thickest near the diaphysis then gradually tapers toward the ends of the bone [38]. Radiographs may also show cupping or splaying of the metaphyses and premature asymmetric closure of the physis, leading to the appearance of a coned epiphysis [34]. Hypervitaminosis A may also cause tendon and ligamentous calcification, including hyperostosis of the anterior cervical spine mimicking DISH [34].
- Voriconazole therapy: Voriconazole is an antifungal medication used for the treatment of severe fungal infections including invasive aspergillosis, esophageal candidiasis, candidemia in neutropenic patients, disseminated Candida skin infections, and Candida infections of wounds and organs [38]. Patients on long term therapy can develop periostitis and present with diffuse bone pain (sometimes resistant to analgesics and narcotics), stiffness, and elevated alkaline phosphatase levels (affecting 15-50% of patients) [38]. Serum fluoride levels are also characteristically elevated [38]. The symptoms usually develop 6 months to 3 years after initiation of treatment [38]. There is a positive correlation between development of periostitis and both higher daily dose and higher cummulative dose [38]. Response to steroids is minimal [38]. Symptoms improve after discontinuation of the medication (typically in 2 weeks to 4 months, but in as few as 2-5 days), although symptoms can also improve with dose reduction [38].
The most commonly affected bones are the ribs, forearms, legs, and shoulders [38]. Involvement of two or more skeletal sites occurs in more than half of the cases [38]. Affected areas show dense, irregular, nodular and usualy bilateral periosteal thickening [38]. On bone scan, the affected areas demonstrate increased tracer uptake [38].
- Progressive diaphyseal dysplasia: Camurati-Engelmann disease is a rare autosomal dominant disorder characterized by bilateral symmetric cortical thickening [34]. The diaphyses of the long bones are expanded due to both endosteal and periosteal new bone formation (the metaphyses and epiphyses are typically spared) [34]. On bone scan, there is increased tracer uptake in the entire thickened cortex, not just along the periosteal surface of the bone [34].
|
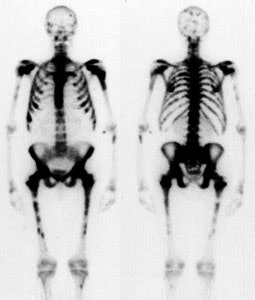
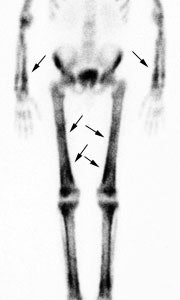
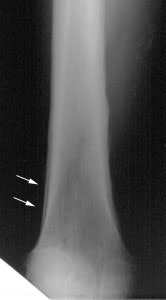
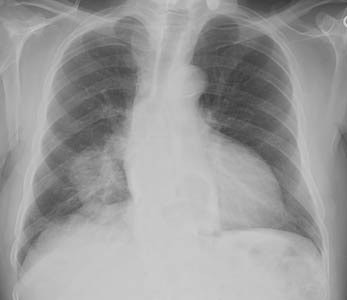
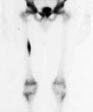
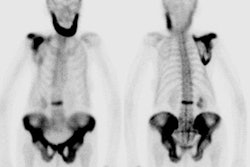
Hypertrophic osteoarthropathy: The patient shown below was being evaluated for bone pain. The bone scan revealed patchy, linear tracer uptake along the femurs, tibias, and distal upper extremitis (black arrows). A plain film reveal a solid periosteal reaction. CXR demonstrated a large right lung mass. |
|
|
Primary Bone Malignancies:
The "Extended pattern" refers to increased activity (usually mild to moderate) in adjacent joints or along the entire ipsilateral extremity in association with a primary tumor of a long bone. This finding may be related to generalized increased blood flow to the extremity. It can lead to over-estimation of the extent of the tumor by scintigraphy. Thallium scintigraphy can be used to better define lesion extent and to monitor response to therapy.
Osteosarcoma:
Osteosarcoma, although a rare lesion, is the most common
primary bone malignancy in children [36]. The most common
location for primary osteosarcoma
is about the knee (50%), followed by the proximal humerus (15%). Common sites for
metastases are the lungs and bones. Although pulmonary mets can be detected in 20% (planar
imaging) to 40% (SPECT imaging) of patients, skeletal mets are detected in only 3% at the time
of presentation. Nonetheless, the presence of skeletal mets is associated with a very poor
prognosis and will greatly alter treatment in these patients. A
baseline bone scan is generally recommended.
Treatment consists of multiagent chemotherapy followed by
complete surgical resection [36]. Patient prognosis depends in
part on the response to chemotherapy [36]. Tumor necrosis of
greater than 90% after therapy generally predicts a good outcome
[36].
Scinitgraphy is much less sensitive
than CT in the detection of pulmonary metastases (with single
headed tomography detecting only 8% of lesions identified by CT
in one series [4]. Although tracer uptake within a pulmonary
lesion is very specific for a metastatic focus of osteosarcoma, the additional cost of tomographic images to detect lung
lesions is difficult to justify, given its lack of sensitivity
compared to CT. The use of follow-up bone scans to detect
metastatic lesions is controversial. Some authors feel that
skeletal mets do not occur in the
absence of lung metastases and therefore serial bone scans are
not indicated unless new pulmonary lesions are detected on
follow-up CT scanning [5]. Others feel bone metastases may be
identified before lung mets;
possibly due to changes in the natural course of the malignancy
in the face of aggressive chemotherapeutic management [6]. In
this case, and in the detection of other unsuspected remote
sites of metastatic disease, routine followup
scintigraphy may be beneficial [7].
PET imaging in osteosarcoma: PET imaging can be used to
evaluate for metabolic tumor response [36]. A change in SUV max
from baseline to week 10 imaging of 60% of more was predictive
of a histologic tumor response [36]. A tumor SUVmax of 4 at week
5, or 3.15 at week 10 were also predictive of tumor response
[36]. In another meta-analysis, an SUVmax of less than 2.5 or a
SUVmax pre- to post therapy ratio of less than or equal to 0.5
were predictive of a histologic response to chemotherapy [36].
Ewing's Sarcoma:
Ewing sarcoma is postulated to arise from embryonal neural
crest cells [32]. Tumors in this group contain a balanced
reciprocal translocation between chromosomes 11 and 22, t(11;22)
(q24;q12) [32]. Over 80% of the Ewing's sarcomas occur in
patients under 20 years of age, and interestingly blacks are
very rarely affected. The tumor usually arises in the diaphysis of a long bone, but 20% arise
in the pelvis and lesions in the pelvis are associated with a
worse prognosis. Lesions arising in the chest wall typically
affect the ribs, scapulae, clavicles, and sternum [32]. Ewing
sarcoma is the most common primary chest wall malignancy in
children and young adults [32].
The most common presenting symptoms are pain and swelling. Patients may complain of systemic symptoms such as fever, anorexia, weight loss, and fatigue, and the clinical presentation may be confused with chronic osteomyelitis [8]. The lesion usually produces a "moth-eaten" pattern of bone destruction on plain film radiographs (76-82% of lesions) with an associated aggressive periosteal reaction [25]. the lesion is typically "hot" on blood pool and delayed bone scan images. FDG PET also shows increased uptake in Ewing's sarcoma and is superior to bone scan for the detection of osseous metastases [25]. The incidence of skeletal mets at the time of presentation ranges from 10 to 40%- most commonly to the lungs and bone [8]. On follow-up, the skeleton is the first site of metastatic disease in about 25% of patients. Baseline, and follow-up bone scans are therefore very useful in these patients.
|
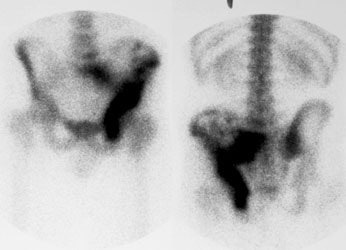
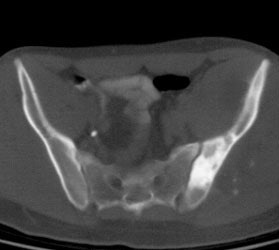
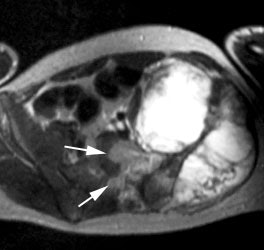
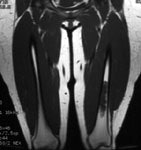
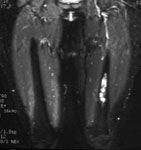
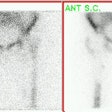
Ewings sarcoma of the pelvis: The bone scan demonstrates extensive, intense tracer uptake involving the left iliac wing, extending into the ischium and left sacrum. CT scan revealed a mixed, but predominantly sclerotic lesion involving the bone with an associated soft tissue mass. Note that the sacrum fails to demonstrate a CT abnormality. The T2 weighted images from the patients MR exam more clearly defines the lesion. Sacral involvement is clearly evident (white arrows) and there is also a large soft tissue component. |
|
|
Benign Bone
Tumors:
Aneurysmal Bone Cyst:
Aneurysmal bone cysts (ABC) may also involve the posterior elements of the spine (20-35% of cases) [8]. Most of these lesions occur in patients less than 20 years of age [8]. On plain film, an ABC appears as an expansile lytic lesion with a very thin or expanded margin and the lesion may involve more than one contiguous vertebrae [8]. Trabeculations can be seen within the lesion. When the lesion grows rapidly, the reparative process in the bone may lag behind, and the margin of the lesion can appear irregular or interrupted.
On CT or MRI, fluid-fluid levels can be identified in 30% of lesions (although this finding can be seen in other, more aggressive bone lesions as well). On bone scan, increased activity is seen within the lesion on all 3 phases. The site of scintigraphic abnormality corresponds to the actual extent of the lesion (extended uptake is not common) [8]. About two-thirds of the lesions will be photopenic centrally, surrounded by a "ring" of increased activity [8].
Bone Island
(Enostosis):
An enostosis is a benign osseous lesion that consists of a
focal area of mature compact (cortical) bone within the
cancellous bone (spongiosa) [31]. They are most frequently found
in the spine, pelvis, and epiphyses or metaphyses of the long
bones [35]. About 30% of bone islands will demonstrate increased
uptake on bone scan- particularly large lesions. Becaused they
are composed of cortical bone, enostoses are expected to be
homogeneously as dense as cortical bone [35]. It has been
suggested that on CT, a mean attenuation above 885 HU and a max
attenuation above 1060 HU provide reliable thresholds for
distinguishing an enostosis from a sclerotic bone metastasis
[31]. Enostoses will often show a spiculated or thorny margin
[35]. On serial imaging, as many as 31% of enostoses can slowly
change in size over time [35].
Cortical desmoid:
Cortical desmoids (distal femoral cortical irregularities) are small (1-3 cm) fibrous or fibroosseous lesions located on the posteromedial surface of the distal supracondylar femur at the site of attachment of the extensor tendinous fibers of the adductor magnus muscle or at the insertion of the medial head of the gastrocnemious tendon [19,20]. These lesions occur in growing children as a result of repetitive traction microavulsions with subsequent fibroblastic response [19]. They are most prevalent in boys aged 10-15 years [20]. The lesion can be bilateral and is usually larger in the dominant extremity [20]. On plain films cortical desmoids appear as an irregularity or defect along the posterior cortex of the femur [20]. The lesion can be hot on bone scan and on FDG PET imaging [19]. On MR- bone marrow edema, periostitis, and edema and swelling of the adjacent soft tissues likely related to remodeling following tendon traction injury [20].
Enchondroma:
Enchondroma is a benign neoplasm of the medullary canal composed of mature hyaline cartilage [16]. it is one of the most common bone lesions- representing 12-24% of all benign bone tumors [16]. Enchondroma and intramedullary chrondrosarcoma can be difficult to distinguish on imaging- however, lesion size, degree of endosteal scalloping, a rim of surrounding edema, and pain favor the diagnosis of intramedullary chondrosarcoma [16]. Enchondromas can have a very variable appearance on bone scan demonstrating very mild, to rather prominent uptake of tracer. MRI can be used to confirm the presence of a chondroid matrix within the lesion if plain films are not conclusive. The high water content of hyaline cartilage produces high signal intensity on T2-images [21].
|
Enchondroma: The patient shown below was being evaluated to exclude a right tibial stress fracture. An unsuspected long segment lesion with very prominent tracer activity was detected in the left distal femoral diaphysis. The patient had no symptoms referable to this lesion. MR images revealed the characteristic appearance of an enchondroma with very bright signal on T2-fat suppressed images (right) due to the chondroid matrix within the lesion (click MR images to enlarge) |
|
|
Multiple enchondromatosis is refered to as Ollier disease and Maffucci syndrome is a variant of multiple enchondromatosis with additonal vascular malformations [27]. Maffucci syndrome is associated with a much higher rate of malignant degeneration [27].
Fibrous dysplasia
In fibrous dysplasia osteoblasts fail to undergo normal morphologic differentiation and maturation leading to the replacement of normal marrow and cancellous bone my immature bone and fibrous stroma [21]. Appromimately 6-20% of monostotic cases affect a rib, and 55% of polyostotic cases will demonstrate rib involvement [21]. On plain film the lesion demonstrates expansile remodeling of the affected bone with a ground glass matrix [21]. On CT, the matrix may appear purely osteolytic or demonstrate peripheral trabeculation [21].
McCune-Albright syndrome is a nonhereditary phakomatosis that
primarily affects females and is characterized by the triad of
polyostotic fibrous dysplasia, cafe-au-lait macules, and
endocrine dysfunction [26]. Fibrous dysplasia typically
manifests during childhood and commonly invovles the skull and
face (50% of cases), pelvis, femur, and tibia [26]. Malignant
degeneration can occur with development of osteosarcoma in 4% of
cases, which is 8 times the rpevalence in isolated monoostotic
fibrous dysplasia [26]. The characteristic skin lesions are
large segmental cafe-au-lait macules which manifest as large tan
patches with jagged "coast of maine" edges that follw lines of
ectoderma migratoion (lines of Blaschko) [26]. The lesions tend
to be unilateral, occuring ipsilateral to skeletal lesions, and
classically do not cross midline [26]. They are most often fond
in the lumbosacral area and on the buttocks [26]. The most
common endocrinologic abnormality is precocious puberty which
occurs more commonly in females (65-79% of cases) than in males
(15%) [26].
On FDG PET imaging, fibrous dysplasia can range from intensely
hypermetabolic to non-FDG avid, and there can be varying degrees
of FDG uptake within different regions of a single lesion or in
different lesions in the same individual [30]. This may be
related to different numbers of proliferating fibroblasts [30].
Higher SUVs are typically seen with increasing number of bone
lesions and in younger patients [30].
Langerhan's Cell Histiocytosis:
Langerhans' Cell Histiocytosis (LCH - formerly referred
to as Histiocytosis X) comprises a
group of reticuloendothelioses
which are not truly neoplasms but
which are characterized by a varied and abnormal proliferation
of dendritic cells [42].
LCH predominantly affects children younger than 15 years of age
[42].
The spectrum of clinical and pathologic entities in this diverse group of diseases include:
1- Unifocal localized form: This accounts for 70% of cases
[28]. There isolated bone involvement (solitary or multifocal).
This was previously referred to as Eosinophilic
Granuloma. Affected patients are
usually between the ages of 5 to 15 years [28].
2- Multifocal unisystem (previously Hand -Schuller-Christian disease): This
accounts for about 20% of cases and invovles multiple bones, as
well as the reticuloendothelial system (liver, spleen, lymph
nodes, and skin) [28]. Affected patients are usually between the
ages of 1 to 5 years [28]. It can be accompanied by diabetes
insipidus if there is pituitary involvement [28]. The
Hand-Schuller-Christian triad consists of lytic bone lesions,
diabetes incipidus, and exophthalamos [42].
3- Multifocal multisystem (previously Letterer-Siwe disease): This accounts for about
10% of cases [28]. Patients usually present in the first 2 years
of life with disseminated involvement of the reticuloendothelial
system, anemia, and thrombocytopenia [28]. The condition is
often fatal [28].
Bone lesions are the most common manifestation of LCH and occur
in approximately 80% of patients [28]. The lesions are most
commonly unifocal (70% of cases) [42]. Approximately 70% of
lesions are found in the flat bones such as the skull, mandible,
pelvis, and ribs [5]. About 30% of lesions involve long bones
[5] (long bone involvement is more common in children than in
adults [28]). The bone lesions can manifest clinically with
point tenderness and swelling, but may also be asymptomatic
[42]. Vertebral body involvement can result in collpase of the
vertebral body (vertebra plana) [28]. LCH is the most common
cause of vertebral plana in children [42].
The typical radiographic appearance of Langerhans' Cell Histiocytosis (LCH) is that of a lucent lesion, sometimes well circumscribed, often with a sclerotic border and a beveled edge. Characteristic beveled lesions occur in the skull and the ileum because of differential destruction of the inner and outer table of the skull and the anterior and posterior surfaces of the ileum. Calvarial bone lesions lack a periosteal reaction [42]. Other radiographic appearances of LCH on skeletal surveys include sclerotic lesions, mixed lytic and sclerotic lesions and complicated lesions such as those involving vertebral compression fractures.
On scintigraphy, up to 30 to 40% of skeletal lesions may not be detected due to their radiolytic nature, and 10% may appear cold [5]. Nonetheless, bone scan is considered by some to be complimentary to skeletal radiography in this disorder. ROC analysis shows that skeletal scintigraphy has the greatest diagnostic accuracy in the skull, facial bones and mandible (88% sensitivity and 52% specificity). The sensitivity of skeletal scintigraphy for detection of lesions in the pelvis, sacrum, ribs, sternum, scapula, and clavicle is poor [3]. On skeletal scintigraphy, LCH typically appears as a focus of intense radiotracer uptake or alternatively as a circumscribed rim of increased radiotracer activity surrounding a central region of photopenia.. After local radiotherapy or systemic chemotherapy, the radiotracer distribution is typically more diffuse and often less avid [3].
In a retrospective study of 56 patients treated at the Mayo
Clinic during 1965-1994 with a definite lesion on radiographs
and biopsy-proven LCH, Howarth et
al reported the sensitivity and specificity of the radiographic
skeletal survey of 100% and 61%, respectively, compared to 91%
and 55% for bone scintigraphy. For
solitary lesions, radiographic sensitivity and specificity were
95% and 73%, respectively, compared with 88% and 77% for bone scintigraphy. These authors support the
use of the radiographic skeletal survey in the diagnosis and
staging of LCH, but suggest that scintigraphy
may be useful in monitoring the response of bone lesions to
treatment, particularly in the case of solitary bone lesions
[3].
Skin involvement occurs in 50% of patients with LCH (isolated
skin involvement is uncommon and occurs in only about 10% of
patients) [42]. Skin lesions have a variable appearance from
nodular to papular and can demonstrate mildly increased tracer
uptake on PET imaging [42].
Organ involvement of the liver, spleen (involvement is less
common than hepatic involvement), or bone marrow is associated
with a worse prognosis [42].
The CNS is affected in 5-10% of cases of LCH and diabetes
incipidus due to infiltration of the pituitary stalk is the most
common manifestation [42].
Non-ossifying fibroma:
Non-ossifying fibromas are well-circumscribed fibrous proliferations that occur most commonly in the juxtaepiphyseal regions of long bones [14]. The most common site is the femur, followed by the tibia [14]. The lesion is found most commonly in children and can occur in as many as 35% of all children [14]. The lesion is usually asymptomatic and discovered incidentally [14]. The lesion usually regresses spontaneously [14].
On x-ray, the lesion appears as an eccentric radiolucent lesion within the cortex of the involved bone [14]. There is often a sclerotic margin and thinning of the overlying cortex [14]. Over time, there is increasing sclerosis/ossification of the lesion extending from its diaphyseal aspect [14]. On CT, the overlying cortex may appear disrupted [14]. On bone scan, the lesion typically shows minimal or no increased tracer uptake [14]. However, lesions that are ossifying may become quite active on bone scan during adolescence [14]. Increased activity within the lesion may also be seen on PET scan (SUV 1.5) [14].
Osteoblastoma:
Osteoblastomas can also involve the posterior elements of the spine. The lesions are typically larger than 2 cm and possess both sclerotic and lytic features [8]. Plain films typically demonstrate an expansile lytic lesion which contains a calcific matrix. On bone scan, the lesion will be hot on both blood pool and delayed images [8].
Osteoid Osteoma:
Osteoid osteomas comprise 10-12% of all
benign osseous neopalsms and 2-3% of all primary bone tumors
[22]. Osteoid osteomas
occur most commonly in adolescents and young adults (7-25 years
[18]), with 90% of cases seen in patients under age 25. There is
a male predominance (1.6:1 to 4:1) [17,22] and the lesion is
significantly more common among white patients [22]. The
lesion can be cortical (most common-75% of cases), medullary (20% of cases), or subperiosteal/periosteal (fewer than 5% of
cases) [22]. Medullary and subperiosteal lesions are
most common within the femoral neck and within the hands and
feet [22]. These two subgroups are also the most common to be
found in intra-articular or juxta-articular locations [22].
The most characteristic clinical presentation is pain
(typically deep, aching, and intense [22]), which is worse at
night and relieved by aspirin or other nonsteroidal
antiinflammatory drug [17]. Symptoms
may last for weeks or months before diagnosis and may also
precede imaging manifestations [22]. If the lesion occurs within
a joint capsule, it may cause joint swelling, synovitis, and restricted mobility [17].
When the lesion occurs in the spine (10-20% of cases), it most
commonly involves the lumbar spine, nearly always occurs in the
posterior elements (50%; only 10% of lesions involve the
vertebraql body), and is frequently associated with a painful
scoliosis with the convexity of the curve oriented away from the
side of the lesion [8,22]. The most common location for a spinal
lesion is the lumbar spine [8].
The most common location for the lesion is the lower
extremities- more than 50% occur in the femur or tibia [22]. The
majority of the tumors involve the cortex of long bones-
typically in the diaphysis or metadiaphysis [22]. Two-thirds of
the femoral lesions are situated in the intertrochanteric or
intracapsular regions of the hip [22].
Osteoid osteomas can be treated medically with NSAIDs,
surgically, or with percutaneous management [22]. Several
authors have reported the natural course of osteoid osteomas is
spontaneous regression and NSAIDs may accelerate this process
[22]. En bloc surgical resection can also be performed with high
success rates (88-97%), but carries surgical risks [22]. Image
guided drill excision and RFA have also been used to treat the
lesion successfully with less morbidity than surgery [22].
On plain films, the nidus of the
lesion (which is highly vascular) is lucent, usually less than
1.5-2.0 cm, and is surrounded by a zone of dense, reactive
fusiform sclerosis [22]. Central calcification may occasionally
be visible within the radiolucent focus [22]. The surrounding
sclerosis may be so profound as to obscure the lucent nidus
[22]. In contrast to corticla lesions, medullary osteoid
osteomas typically produce mild-to-moderate eccentric sclerosis
[22]. Subperiosteal lesions manifest as soft tissue maasess
adjacent to the affected bone and often do not produce reactive
sclerosis [22]. The bone subajacent to lesion reveals irregular
bony resorption [22].
CT is the diagnostic modality of choice for tumor detection
[22]. CT is more accurate than MR for the detection of the tumor
nidus [22]. At CT, the nidus is
will defined low attenuation round
or oval focus with surrounding reactive sclerosis [18]. MRI
demonstrates decreased signal intensity due to the reactive bony
sclerosis, but the nidus may
produce a brighter signal. Edema in the adjacent bone marrow and
soft tissues and a joint effusion may also be seen [18].
On MRI, the tumor exhibits low-to-intermediate signal on T1
images and heterogenenously high signal on T2 and STIR sequences
[22]. Tumor enhancement is variable, but most lesions enhance
diffusely as a result of intrinsic vascularity [22]. The
surrounding sclerosis manifests as fusiform low signal on both
T1 and T2 images [22]. MRI can also demonstrate adjacent marrow
and soft tissue edema [22], synovitis, and joint effusion [29].
On bone scan, the lesion is hot on all 3-phases. On delayed images the nidus(a focus of very high bone turnover) may be seen as a focal area of even greater activity within the diffuse abnormality- this produces a "double-density sign" [17,22]. The sensitivity of bone scan for the detection of osteoid osteomas is virtually 100% [22]. Intraoperative localization of the lesion and nidus can be accomplished through the use of a hand held probe. A normal bone scan virtually excludes the diagnosis of an osteoid osteoma.
|
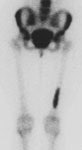
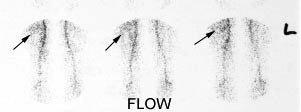
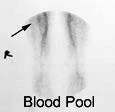
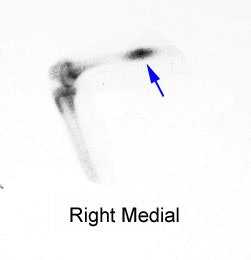
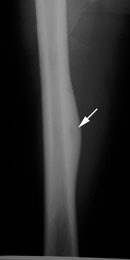
Right femoral osteoid osteoma: Flow (click to enlarge) and blood pool images demonstrated a focus of increased activity within the right mid-femoral diaphysis (black arrows). Intense tracer accumulation could be seen in this region on the delayed images. A delayed right medial spot image demonstrated a focal area of even greater activity (blue arrow) within the diffuse abnormality corresponding to the lesions nidus. Plain film (click to enlarge) demonstrates the characteristic appearance of an osteoid osteoma with a dense region of reactive sclerosis surrounding a central area of lucency (white arrow) (click image to enlarge) |
|
|
Giant cell tumor:
Giant cell tumors of bone are benign mesenchymal tumors
composed of mononuclear stomal cells and characteristic
multinuclear giant cells [33]. The lesion is most commonly
located in the epiphyses of long bones with 50% localized at the
distal femur, proximal tibia, or distal radius [33]. Peak
incidence is in the 3rd-4th decade of life [33]. On x-ray, GCTs
appear as lytic with well defined nonsclerotic margins [33].
Traditionally, treatment of GCT was surgical resection or
curettage [33]. Denosumab is a new monoclonal antibody
antagonist of the recptor activator of nuclear factor kappa B
ligand (RANKL), a surface protein involved in bone homeostasis,
that has shown promising results in the treatment of GCT [33].
The tumor demonstrates tracer uptake on FDG PET imaging and
intense thallium-201 accumulation [33].
REFERENCES:
(1) J Nucl Med 1985; Tumeh SS, et al. Clinical significance of solitary rib lesions in patients with extraskeletal malignancy. 26: 1140-1143
(2) J Nucl Med 1990; Jacobson AF, et al. Association between number and sites of new bone scan abnormalities and presence of skeletal metastases in patients with breast cancer. 31: 387-392
(3) Radiol Clin North Am 1993; Brown ML. Bone scintigraphy in benign and malignant tumors. 31(4):731-8. Review.
(4) AJR 1984; Vanel D, et al. Pulmonary evaluation of patients with osteosarcoma: roles of standard radiography, tomography, CT, scintigraphy, and tomoscintigraphy. 143: 519-523
(5) Skeletal Radiol 1986; Rees CR, et al. The role of bone scintigraphy in osteogenic sarcoma. 15: 365-367
(6) Radiology 1980; 135: 177-180
(7) J Nucl Med 1990; Arrington ER, at al. Scintigraphic appearance of uncommon soft-tissue osteogenic sarcoma metastases. 31: 679-681
(8) Semin Nucl Med 1993; Sty JR, et al. Spine pain in children. 23: 296-320
(9) Cancer Control 1998; Mayak MJ. Clinical applications of radioimmunoscintigraphy with prostate-specific antibodies for prostate cancer. 5: 493-499
(10) Radiographics 2003; Love C, et al. Radionuclide bone imaging: an illustrative review. 23: 341-358
(11) J Nucl Med 2005; Even-Sapir E. Imaging of malignant bone involvement by morphologic, scintigraphic, and hybrid modalities. 46: 1356-1367
(12) AJR 2006; Flare response in 18F-fluoride ion PET bone scanning. 186: 1783-1786
(13) J Nucl Med 2006; Romer W, et al. SPECT-guided CT fot evaluating foci of increased bone metabolism classified as indeterminate on SPECT in cancer patients. 47: 1102-1106
(14) AJR 2006; Iagaru A, Henderson R. PET/CT followup in nonossifying fibroma. 187: 830-832
(15) Radiology 2007; Hricak H, et al. Imaging prostate cancer: a multidisciplinary perspective. 243: 28-53
(16) AJR 2008; Walden MJ, et al. Incidental enchondromas of the knee. 190: 1611-1615
(17) Radiographics 2009; Motamedi D, et al. Thermal ablation of osteoid osteoma: overview and step-by-step guide. 29: 2127-2141
(18) Radiographics 2010; Chai JW, et al. Radiologic diagnosis of osteoid osteoma: from simple to challenging findings. 30: 737-749
(19) AJR 2006; Goodin GS, et al. PET/CT characterization of fibroosseous defects in children: 18F-FDG uptake can mimic metastatic disease. 187: 1124-1128
(20) AJR 2011;La Rocca Vieira R, et al. MRI features of cortical desmoid in acute knee trauma. 196: 424-428
(21) Radiographics 2011; Nam SJ,
et al. Imaging of primary chest wall
tumors with radiologic-pathologic correlation. 31: 749-770
(22) AJR 2012; mIyer RS, et al. Pediatric bone imaging:
diagnostic imaging of osteoid osteoma. 198: 1039-1052
(23) J Nucl med 2012; Saylor PJ, et al. Multitargeted tyrosine
kinase inhibition produces discordant changes between 99Tc-MDP
bone
scans
and
other
disease biomarkers: analysis of a phase II study of sunitinib
for metastatic castration-resistant prostate cancer. 53:
1670-1675
(24) J Nucl Med 2013; Wong KK, Piert M. Dynamic bone imaging
with 99mTc-labeled diphosphonates and 18F-NaF:
mechanisms and applications. 54: 590-599
(25) Radiographics 2013; Murphey MD, et al. From the radiologic
pathology archives. Ewing sarcoma family of tumors:
radiologic-pathologic correlation. 33: 803-831
(26) Radiographics 2013; Lew PP, et al. Imaging of disroders
affecting the bone and skin. 34: 197-216
(27) Radiographics 2013; Lew PP, et al. Imaging of disorders
affecting the bone and skin. 34: 197-216
(28) Radiographics 2014; Zaveri J, et al. More than just
Langerhans cell histiocytosis: a radiologic review of
histiocytic disorders. 34: 2008-2024
(29) AJR 2015; Klontzas ME, et al. Osteoid osteoma of the
femoral neck: use of the half-moon sign in MRI diagnosis. 205:
353-357
(30) Radiographics 2016; White ML, et al. Specturm of benign
articular and periarticular findings at FDG PET/CT. 36: 824-839
(31) AJR 2016; Ulano A, et al. Distinguishing untreated
osteoblastic metastases from enostoses using CT attenuation
measurements. 207: 362-368
(32)Radiographics 2016; Carter BW, et al. Imaging evaluation of
malignant chest wall neoplasms. 36: 1285-1306
(33) AJR 2017; Keller S, et al. Thallium-201 uptake of giant
cell tumor: one step toward the differential diagnosis to
atypically presenting osteosarcoma. 208: 171-179
(34) Radiographics 2017; Yap FY, et al. Hypertrophic
osteoarthropathy: clinical and imaging features. 37: 157-175
(35) AJR 2017; Bernard S, et al. An approach to the evaluation
of incidentally identified bone lesions encountered on imaging
studies. 208: 960-970
(36) J Nucl Med 2018; Davis JC, et al. 18F-FDG
uptake during early adjuvant chemotherapy predicts histologic
response in pediatric and young adult patients with
osteosarcoma. 59: 25-30
(37) Radiology 2018; Park SY, et al. Gallium 68 PMSA-11 PET/MR
imaging in patients with intermediate- or high risk prostate
cancer. 288: 495-505
(38) AJR 2019; Tan I, et al. Spectrum of voriconazole-induced
periostitis with review of the differential diagnosis. 212:
157-165
(39) Radiology 2019; Perez-Lopez R, et al. Imaging diagnosis
and follow-up of advanced prostate cancer: clinical perspectives
and state of the art. 292: 273-286
(40) J Nucl Med 2020; Pomykala KL, et al. 68Ga-PMSA-11
PET/CT for bone metastases detection in prostate cancer
patients: potential impact on bone scan guidelines. 61: 405-411
(41) J Nucl Med 2020; Cook GJR, Goh V. Molecular imaging of
bone metastases and their response to therapy. 61: 799-806
(42) Radiographics 2021; Huynh KN, Nguyen BD. Histiocytosis and
neoplasms of the macrophage-dendritic lineages: multimodality
imaging with emphysis on PET/CT. 41: 576-594